Label: CARNITOR- levocarnitine tablet
- NDC Code(s): 54482-144-07
- Packager: Leadiant Biosciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
CARNITOR® (levocarnitine) is a carrier molecule in the transport of long-chain fatty acids across the inner mitochondrial membrane.
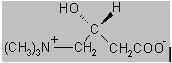
The chemical name of levocarnitine is 3-carboxy-2(R)-hydroxy-N,N,N-trimethyl-1-propanaminium, inner salt. Levocarnitine is a white crystalline, hygroscopic powder. It is readily soluble in water, hot alcohol, and insoluble in acetone. The specific rotation of levocarnitine is between -29° and -32°. Its chemical structure is:
Empirical Formula: C7H15NO3
Molecular Weight: 161.20
Each CARNITOR® (levocarnitine) Tablet contains 330 mg of levocarnitine and the inactive ingredients magnesium stearate, microcrystalline cellulose and povidone.
Each 118 mL container of CARNITOR® (levocarnitine) Oral Solution contains 1 g of levocarnitine/10 mL. Also contains: Artificial Cherry Flavor, D,L,-Malic Acid, Purified Water, Sucrose Syrup. Methylparaben NF and Propylparaben NF are added as preservatives. The pH is approximately 5.
Each 118 mL container of CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution contains 1 g of levocarnitine/10 mL. Also contains: Natural Cherry Flavor, D,L,-Malic Acid, Purified Water, Sodium Saccharin USP. Methylparaben NF and Propylparaben NF are added as preservatives. The pH is approximately 5.
-
CLINICAL PHARMACOLOGY
CARNITOR® (levocarnitine) is a naturally occurring substance required in mammalian energy metabolism. It has been shown to facilitate long-chain fatty acid entry into cellular mitochondria, thereby delivering substrate for oxidation and subsequent energy production. Fatty acids are utilized as an energy substrate in all tissues except the brain. In skeletal and cardiac muscle, fatty acids are the main substrate for energy production.
Primary systemic carnitine deficiency is characterized by low concentrations of levocarnitine in plasma, RBC, and/or tissues. It has not been possible to determine which symptoms are due to carnitine deficiency and which are due to an underlying organic acidemia, as symptoms of both abnormalities may be expected to improve with CARNITOR®. The literature reports that carnitine can promote the excretion of excess organic or fatty acids in patients with defects in fatty acid metabolism and/or specific organic acidopathies that bioaccumulate acylCoA esters.1-6
Secondary carnitine deficiency can be a consequence of inborn errors of metabolism. CARNITOR® may alleviate the metabolic abnormalities of patients with inborn errors that result in accumulation of toxic organic acids. Conditions for which this effect has been demonstrated are: glutaric aciduria II, methyl malonic aciduria, propionic acidemia, and medium chain fatty acylCoA dehydrogenase deficiency.7,8 Autointoxication occurs in these patients due to the accumulation of acylCoA compounds that disrupt intermediary metabolism. The subsequent hydrolysis of the acylCoA compound to its free acid results in acidosis which can be life-threatening. Levocarnitine clears the acylCoA compound by formation of acylcarnitine, which is quickly excreted. Carnitine deficiency is defined biochemically as abnormally low plasma concentrations of free carnitine, less than 20 µmol/L at one week post term and may be associated with low tissue and/or urine concentrations. Further, this condition may be associated with a plasma concentration ratio of acylcarnitine/levocarnitine greater than 0.4 or abnormally elevated concentrations of acylcarnitine in the urine. In premature infants and newborns, secondary deficiency is defined as plasma levocarnitine concentrations below age-related normal concentrations.
-
PHARMACOKINETICS
In a relative bioavailability study in 15 healthy adult male volunteers, CARNITOR® Tablets were found to be bio-equivalent to CARNITOR® Oral Solution. Following 4 days of dosing with 6 tablets of CARNITOR® 330 mg b.i.d. or 2 g of CARNITOR® oral solution b.i.d., the maximum plasma concentration (Cmax) was about 80 µmol/L and the time to maximum plasma concentration (Tmax) occurred at 3.3 hours.
The plasma concentration profiles of levocarnitine after a slow 3 minute intravenous bolus dose of 20 mg/kg of CARNITOR® were described by a two-compartment model. Following a single i.v. administration, approximately 76% of the levocarnitine dose was excreted in the urine during the 0-24h interval. Using plasma concentrations uncorrected for endogenous levocarnitine, the mean distribution half life was 0.585 hours and the mean apparent terminal elimination half life was 17.4 hours.
The absolute bioavailability of levocarnitine from the two oral formulations of CARNITOR®, calculated after correction for circulating endogenous plasma concentrations of levocarnitine, was 15.1 ± 5.3% for CARNITOR® Tablets and 15.9 ± 4.9% for CARNITOR® Oral Solution.
Total body clearance of levocarnitine (Dose/AUC including endogenous baseline concentrations) was a mean of 4.00 L/h.
Levocarnitine was not bound to plasma protein or albumin when tested at any concentration or with any species including the human.9
-
METABOLISM AND EXCRETION
In a pharmacokinetic study where five normal adult male volunteers received an oral dose of [3H-methyl]-L-carnitine following 15 days of a high carnitine diet and additional carnitine supplement, 58 to 65% of the administered radioactive dose was recovered in the urine and feces in 5 to 11 days. Maximum concentration of [3H-methyl]-L-carnitine in serum occurred from 2.0 to 4.5 hr after drug administration. Major metabolites found were trimethylamine N-oxide, primarily in urine (8% to 49% of the administered dose) and [3H]-γ-butyrobetaine, primarily in feces (0.44% to 45% of the administered dose). Urinary excretion of levocarnitine was about 4 to 8% of the dose. Fecal excretion of total carnitine was less than 1% of the administered dose.10
After attainment of steady state following 4 days of oral administration of CARNITOR® Tablets (1980 mg q12h) or Oral Solution (2000 mg q12h) to 15 healthy male volunteers, the mean urinary excretion of levocarnitine during a single dosing interval (12h) was about 9% of the orally administered dose (uncorrected for endogenous urinary excretion).
-
INDICATIONS AND USAGE
CARNITOR® (levocarnitine) is indicated in the treatment of primary systemic carnitine deficiency. In the reported cases, the clinical presentation consisted of recurrent episodes of Reye-like encephalopathy, hypoketotic hypoglycemia, and/or cardiomyopathy. Associated symptoms included hypotonia, muscle weakness and failure to thrive. A diagnosis of primary carnitine deficiency requires that serum, red cell and/or tissue carnitine levels be low and that the patient does not have a primary defect in fatty acid or organic acid oxidation (see CLINICAL PHARMACOLOGY). In some patients, particularly those presenting with cardiomyopathy, carnitine supplementation rapidly alleviated signs and symptoms. Treatment should include, in addition to carnitine, supportive and other therapy as indicated by the condition of the patient.
CARNITOR® (levocarnitine) is also indicated for acute and chronic treatment of patients with an inborn error of metabolism which results in a secondary carnitine deficiency.
- CONTRAINDICATIONS
-
WARNINGS
Hypersensitivity Reactions
Serious hypersensitivity reactions, including rash, urticaria, and facial edema have been reported with oral CARNITOR®. Other serious hypersensitivity reactions, including anaphylaxis, laryngeal edema, and bronchospasm have been reported following intravenous levocarnitine administration, mostly in patients with end stage renal disease undergoing dialysis.
Discontinue use of CARNITOR® and instruct patients to seek medical attention if they experience symptoms suggestive of a hypersensitivity reaction.
-
PRECAUTIONS
General
CARNITOR® (levocarnitine) Oral Solution and CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution are for oral/internal use only.
Not for parenteral use.
Gastrointestinal reactions may result from a too rapid consumption of carnitine. CARNITOR® (levocarnitine) Oral Solution and CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution may be consumed alone, or dissolved in drinks or other liquid foods to reduce taste fatigue. They should be consumed slowly and doses should be spaced evenly throughout the day to maximize tolerance.
The safety and efficacy of oral levocarnitine has not been evaluated in patients with renal insufficiency. Chronic administration of high doses of oral levocarnitine in patients with severely compromised renal function or in ESRD patients on dialysis may result in accumulation of the potentially toxic metabolites, trimethylamine (TMA) and trimethylamine-N-oxide (TMAO), since these metabolites are normally excreted in the urine.
Drug Interactions
Reports of INR increase with the use of warfarin have been observed. It is recommended that INR levels be monitored in patients on warfarin therapy after the initiation of treatment with levocarnitine or after dose adjustments.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenicity tests performed in Salmonella typhimurium, Saccharomyces cerevisiae, and Schizosaccharomyces pombe indicate that levocarnitine is not mutagenic. No long-term animal studies have been performed to evaluate the carcinogenic potential of levocarnitine.
Pregnancy
Reproductive studies have been performed in rats and rabbits at doses up to 3.8 times the human dose on the basis of surface area and have revealed no evidence of impaired fertility or harm to the fetus due to CARNITOR®. There are, however, no adequate and well controlled studies in pregnant women.
Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Levocarnitine supplementation in nursing mothers has not been specifically studied.
Studies in dairy cows indicate that the concentration of levocarnitine in milk is increased following exogenous administration of levocarnitine. In nursing mothers receiving levocarnitine, any risks to the child of excess carnitine intake need to be weighed against the benefits of levocarnitine supplementation to the mother. Consideration may be given to discontinuation of nursing or of levocarnitine treatment.
-
ADVERSE REACTIONS
The following adverse reactions associated with the use of oral formulations of levocarnitine were identified in clinical trials or postmarketing reports. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency, reliability, or to establish a causal relationship to drug exposure.
Gastrointestinal Reactions: Various mild gastrointestinal complaints have been reported during the long-term administration of oral L- or D,L-carnitine; these include transient nausea and vomiting, abdominal cramps, and diarrhea. Gastrointestinal adverse reactions with CARNITOR® (levocarnitine) Oral Solution or CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution dissolved in liquids might be avoided by a slow consumption of the solution or by a greater dilution. Decreasing the dosage often diminishes or eliminates drug-related patient body odor or gastrointestinal symptoms when present. Tolerance should be monitored very closely during the first week of administration, and after any dosage increases.
Musculoskeletal Reactions: Mild myasthenia has been described only in uremic patients receiving D,L-carnitine.
Neurologic Reactions: Seizures have been reported to occur in patients with or without pre-existing seizure activity receiving either oral or intravenous levocarnitine. In patients with pre-existing seizure activity, an increase in seizure frequency and/or severity has been reported.
Hypersensitivity Reactions: Rash, urticaria, and facial edema have been reported with oral CARNITOR® (see WARNINGS).
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
CARNITOR® (levocarnitine) Tablets.
Adults: The recommended oral dosage for adults is 990 mg two or three times a day using the 330 mg tablets, depending on clinical response.
Infants and children: The recommended oral dosage for infants and children is between 50 and 100 mg/kg/day in divided doses, with a maximum of 3 g/day. Dosage should begin at 50 mg/kg/day. The exact dosage will depend on clinical response.
Monitoring should include periodic blood chemistries, vital signs, plasma carnitine concentrations and overall clinical condition.
CARNITOR® (levocarnitine) Oral Solution and CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution.
For oral use only. Not for parenteral use.
Adults: The recommended dosage of levocarnitine is 1 to 3 g/day for a 50 kg subject, which is equivalent to 10 to 30 mL/day of CARNITOR® (levocarnitine) Oral Solution or CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution. Higher doses should be administered only with caution and only where clinical and biochemical considerations make it seem likely that higher doses will be of benefit. Dosage should start at 1 g/day, (10 mL/day), and be increased slowly while assessing tolerance and therapeutic response. Monitoring should include periodic blood chemistries, vital signs, plasma carnitine concentrations, and overall clinical condition.
Infants and children: The recommended dosage of levocarnitine is 50 to 100 mg/kg/day which is equivalent to 0.5 mL/kg/day CARNITOR® (levocarnitine) Oral Solution or CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution. Higher doses should be administered only with caution and only where clinical and biochemical considerations make it seem likely that higher doses will be of benefit. Dosage should start at 50 mg/kg/day, and be increased slowly to a maximum of 3 g/day (30 mL/day) while assessing tolerance and therapeutic response. Monitoring should include periodic blood chemistries, vital signs, plasma carnitine concentrations, and overall clinical condition.
CARNITOR® (levocarnitine) Oral Solution or CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution may be consumed alone or dissolved in drink or other liquid food. Doses should be spaced evenly throughout the day (every three or four hours) preferably during or following meals and should be consumed slowly in order to maximize tolerance.
-
HOW SUPPLIED
CARNITOR® (levocarnitine) Tablets are supplied as 330 mg tablets embossed with "CARNITOR ST" in individual blisters, packaged in boxes of 90 (NDC 54482-144-07). Store in original packaging: content hygroscopic. Store at 20°C to 25°C (68°F to 77°F). See USP Controlled Room Temperature. CARNITOR® (levocarnitine) Tablets are distributed by Leadiant Biosciences, Inc.
CARNITOR® (levocarnitine) Oral Solution is supplied in 118 mL (4 FL. OZ.) plastic containers (NDC 54482-145-08 and NDC 54482-145-09). Store at 20°C to 25°C (68°F to 77°F). See USP Controlled Room Temperature. Avoid excessive heat and protect from freezing. CARNITOR® (levocarnitine) Oral Solution is distributed by Leadiant Biosciences, Inc.
CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution is supplied in 118 mL (4 FL. OZ.) plastic containers (NDC 54482-148-01 and NDC 54482-148-02). Store at 20°C to 25°C (68°F to 77°F). See USP Controlled Room Temperature. Avoid excessive heat and protect from freezing. CARNITOR® SF (levocarnitine) Sugar-Free Oral Solution is distributed by Leadiant Biosciences, Inc.
Rx only.
-
REFERENCES
- Bohmer, T., Rydning, A. and Solberg, H.E. 1974. Carnitine levels in human serum in health and disease. Clin. Chim. Acta 57:55-61.
- Brooks, H., Goldberg, L., Holland, R. et al. 1977. Carnitine-induced effects on cardiac and peripheral hemodynamics. J. Clin. Pharmacol. 17:561-568.
- Christiansen, R., Bremer, J. 1976. Active transport of butyrobetaine and carnitine into isolated liver cells. Biochim. Biophys. Acta 448:562-577.
- Lindstedt, S. and Lindstedt, G. 1961. Distribution and excretion of carnitine in the rat. Acta Chem. Scand. 15:701-702.
- Rebouche, C.J. and Engel, A.G. 1983. Carnitine metabolism and deficiency syndromes. Mayo Clin. Proc. 58:533-540.
- Rebouche, C.J. and Paulson, D.J. 1986. Carnitine metabolism and function in humans. Ann. Rev. Nutr. 6:41-66.
- Scriver, C.R., Beaudet, A.L., Sly, W.S. and Valle, D. 1989. The Metabolic Basis of Inherited Disease. New York: McGraw-Hill.
- Schaub, J., Van Hoof, F. and Vis, H.L. 1991. Inborn Errors of Metabolism. New York: Raven Press.
- Marzo, A., Arrigoni Martelli, E., Mancinelli, A., Cardace, G., Corbelletta, C., Bassani, E. and Solbiati, M. 1991. Protein binding of L-carnitine family components. Eur. J. Drug Met. Pharmacokin. Special Issue III: 364-368.
- Rebouche, C.J. 1991. Quantitative estimation of absorption and degradation of a carnitine supplement by human adults. Metabolism 40:1305-1310.
PREVIOUS EDITION IS OBSOLETE
Date of Issue: 06/23
OPI-15 - PRINCIPAL DISPLAY PANEL - 330 mg Tablet Blister Pack Carton
-
INGREDIENTS AND APPEARANCE
CARNITOR
levocarnitine tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54482-144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOCARNITINE (UNII: 0G389FZZ9M) (LEVOCARNITINE - UNII:0G389FZZ9M) LEVOCARNITINE 330 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code CARNITOR;ST Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54482-144-07 10 in 1 CARTON 12/27/1985 1 9 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018948 12/27/1985 Labeler - Leadiant Biosciences, Inc. (068301431)