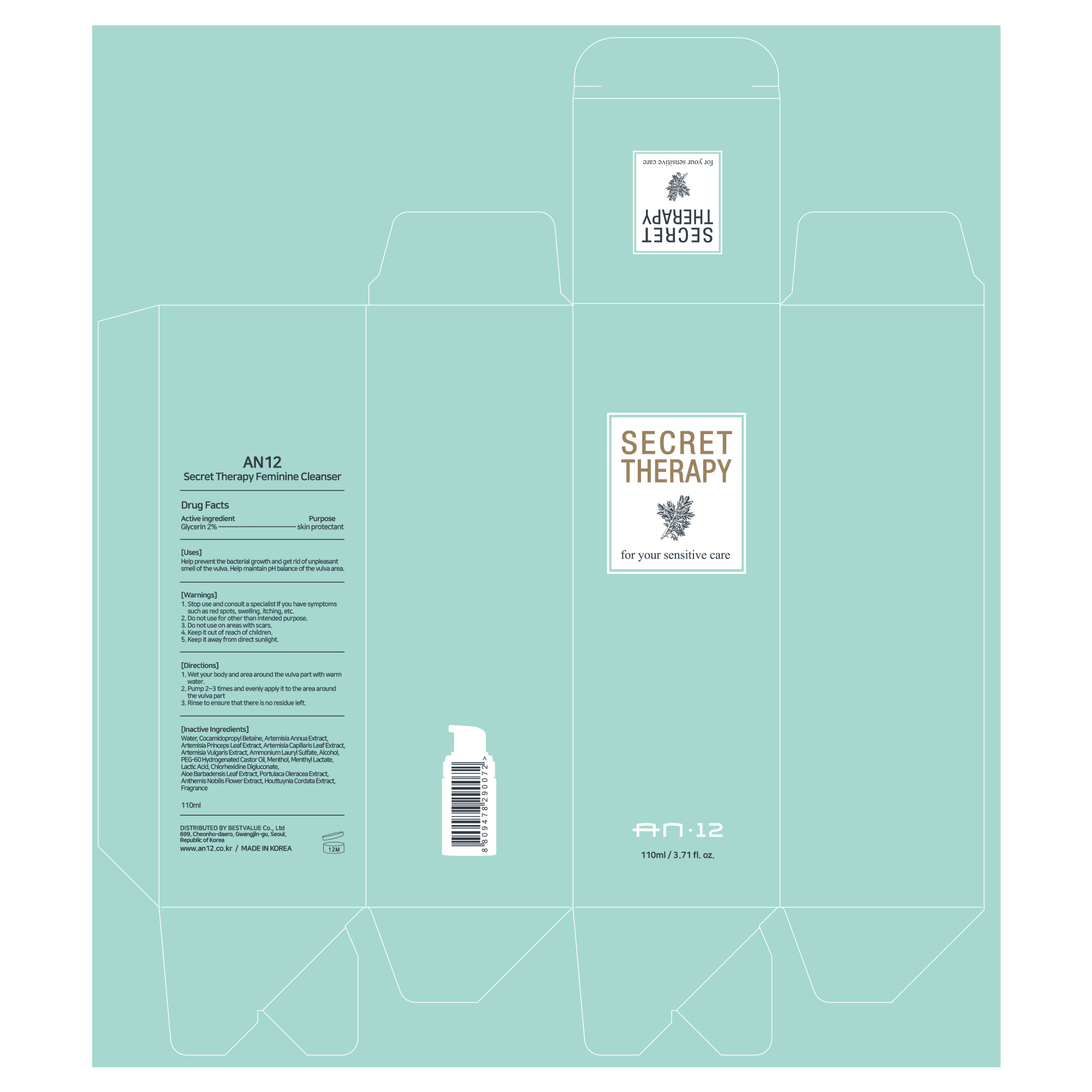
Label: AN12 SECRET THERAPY FEMININE CLEANSER- glycerin liquid
- NDC Code(s): 82385-103-01
- Packager: BESTVALUE Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Warnings
- Warnings
- Warnings
-
Inactive Ingredients
Water, Cocamidopropyl Betaine, Artemisia Annua Extract, Artemisia Princeps Leaf Extract, Artemisia Capillaris Leaf Extract,
Artemisia Vulgaris Extract, Ammonium Lauryl Sulfate, Alcohol, PEG-60 Hydrogenated Castor Oil, Menthol, Menthyl Lactate,
Lactic Acid, Chlorhexidine Digluconate, Aloe Barbadensis Leaf Extract, Portulaca Oleracea Extract, Anthemis Nobilis Flower Extract, Houttuynia Cordata Extract, Fragrance
- Directions
- Display Label
-
INGREDIENTS AND APPEARANCE
AN12 SECRET THERAPY FEMININE CLEANSER
glycerin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82385-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 g in 100 mL Inactive Ingredients Ingredient Name Strength FRAGRANCE FRESH CITRUS FLORAL ORC1501495 (UNII: OU4GI2R2WB) ARTEMISIA ANNUA FLOWERING TOP (UNII: 0UQK6O82OW) ARTEMISIA PRINCEPS LEAF (UNII: SY077EW02G) ARTEMISIA CAPILLARIS LEAF (UNII: KRM0ZYQ69Z) MENTHOL (UNII: L7T10EIP3A) PURSLANE (UNII: M6S840WXG5) ARTEMISIA VULGARIS WHOLE (UNII: JDR81QW9ZQ) POLYOXYL 60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) WATER (UNII: 059QF0KO0R) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) LACTIC ACID (UNII: 33X04XA5AT) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) ALCOHOL (UNII: 3K9958V90M) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82385-103-01 110 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 12/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/01/2021 Labeler - BESTVALUE Co., Ltd (694025785) Establishment Name Address ID/FEI Business Operations BESTVALUE Co., Ltd 694025785 manufacture(82385-103)