Label: PLUSET- porcine follicle stimulating hormone, porcine luteinizing hormone kit kit
- NDC Code(s): 76307-101-01, 76307-201-02, 76307-301-03
- Packager: Laboratorios Calier S.A.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
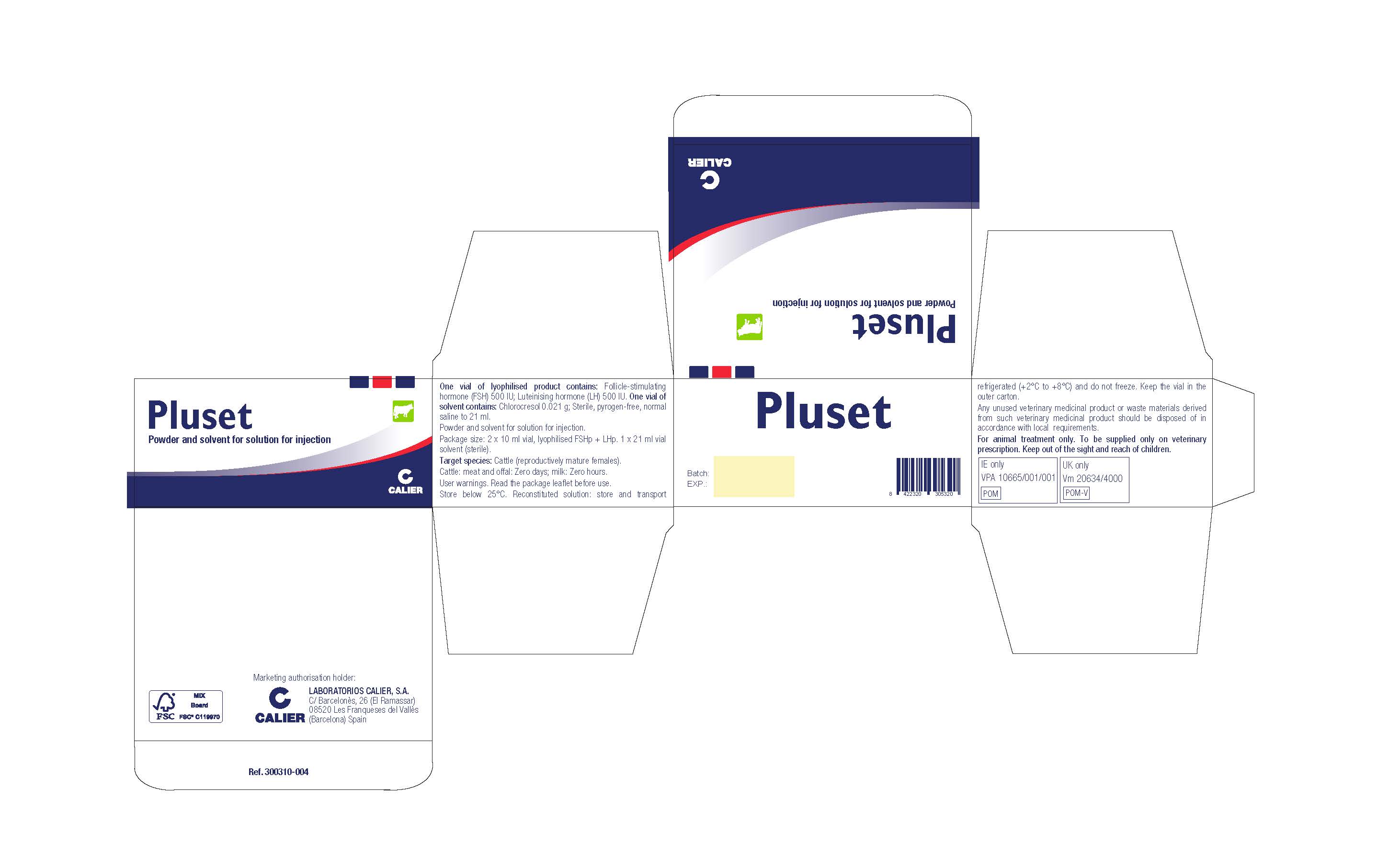
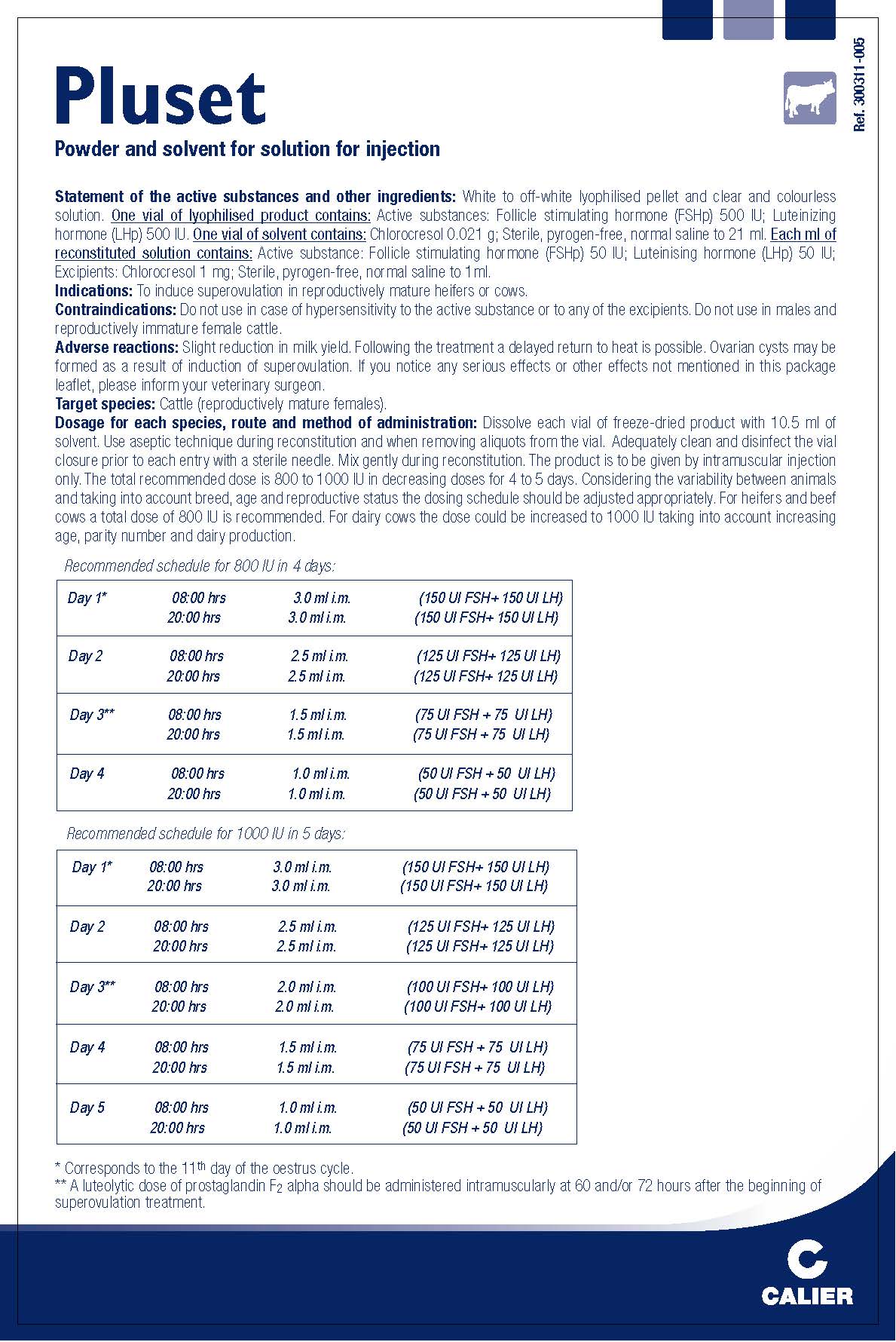
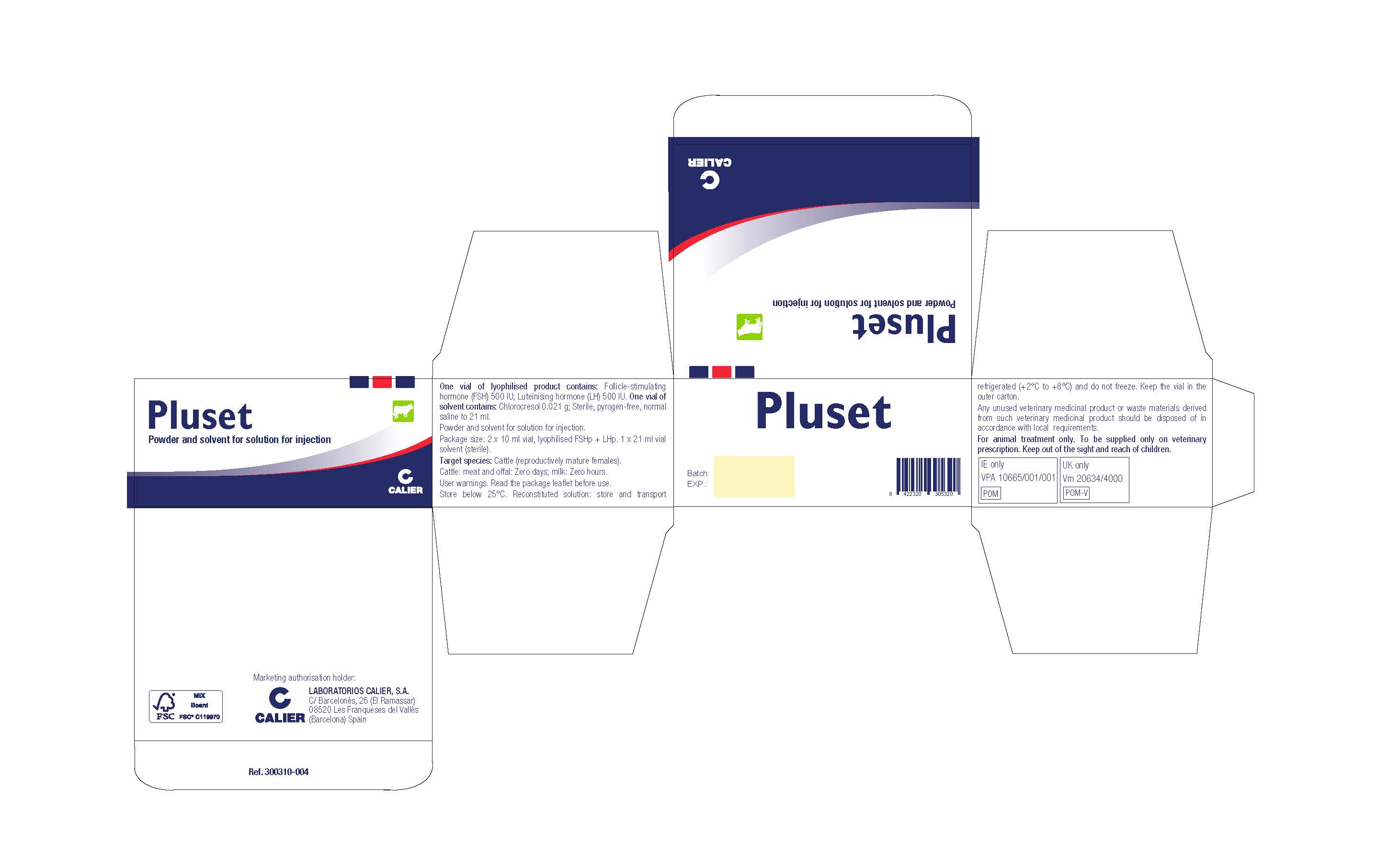
- Indications:
- Contraindications:
- Adverse reactions:
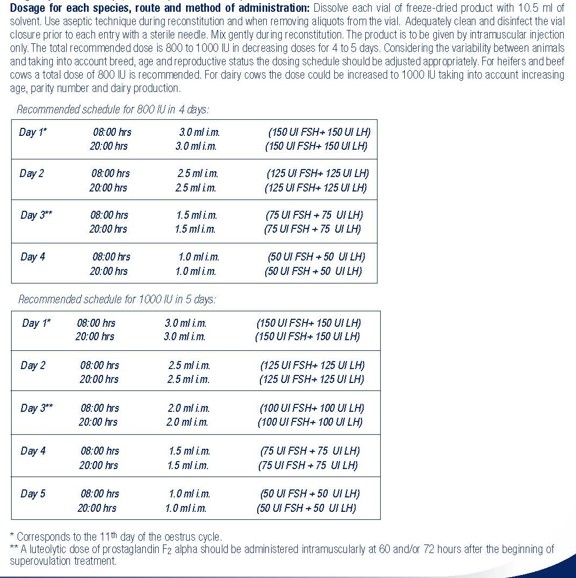
- Dosage for each species, route and method of administration:
- Withdrawal period:
-
Special storage precautions:
Keep out of the sight and reach of children. Store below 25°C. Reconstituted solution: store and transport refrigerated (+2°C to +8°C) and do not freeze. Keep the vial in the outer carton. Do not use this veterinary medicinal product after the expiry date which is stated on the label and carton after EXP. The expiry date refers to the last day of that month. Shelf-life after reconstitution according to directions: 6 days.
- Special Warnings and User Warnings:
- Special precautions for the disposal of unused product or waste materials, if any:
- Other information:
- Package Insert
- PLUSET lyophilized powder vial label
- PLUSET Solvent vial label
- PLUSET Carton Label
-
INGREDIENTS AND APPEARANCE
PLUSET
porcine follicle stimulating hormone, porcine luteinizing hormone kit kitProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:76307-101 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76307-101-01 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL, MULTI-DOSE 20 mL Part 2 1 VIAL, GLASS 21 mL Part 1 of 2 PLUSET
follicle stimulating hormone, luteinizing hormone injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:76307-201 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOLLICLE STIMULATING HORMONE (PORCINE) (UNII: 8FYM5179QJ) (FOLLICLE STIMULATING HORMONE (PORCINE) - UNII:8FYM5179QJ) FOLLICLE STIMULATING HORMONE (PORCINE) 50 [iU] in 1 mL LUTEINIZING HORMONE (UNII: 8XA4VN1LH4) (LUTEINIZING HORMONE - UNII:8XA4VN1LH4) LUTEINIZING HORMONE 50 [iU] in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76307-201-02 10 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/10/2024 Part 2 of 2 PLUSET SOLVENT
physiological saline injectionProduct Information Item Code (Source) NDC:76307-301 Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength CHLOROCRESOL (UNII: 36W53O7109) 0.021 g in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76307-301-03 21 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/10/2024 Labeler - Laboratorios Calier S.A. (460009038) Establishment Name Address ID/FEI Business Operations Laboratorios Calier S.A. 460009038 analysis, manufacture, api manufacture, pack, label