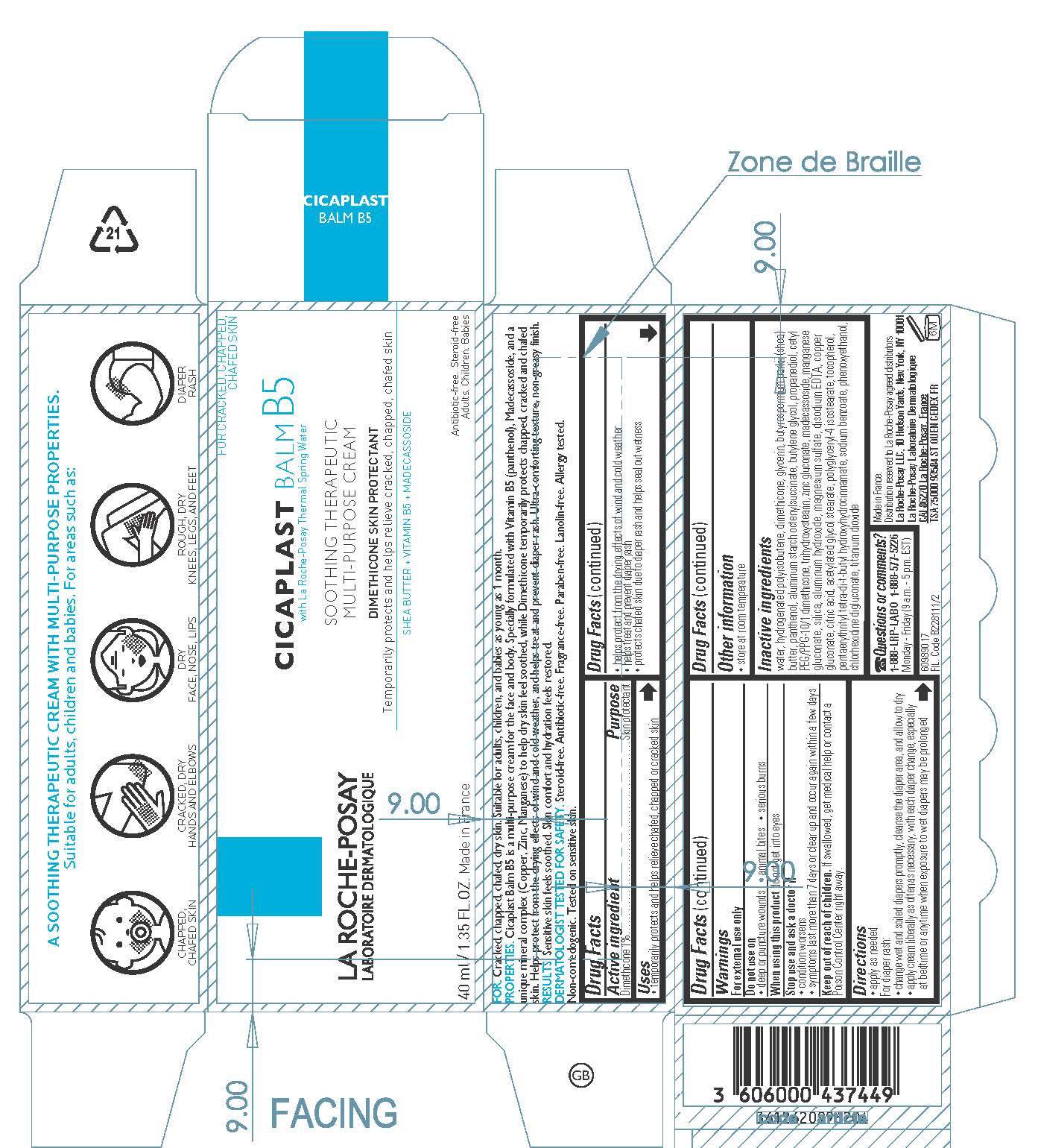
Label: LA ROCHE POSAY LABORATOIRE DERMATOLOGIQUE CICAPLAST BALM B5 THERAPEUTIC MULTIPURPOSE- dimethicone cream
- NDC Code(s): 69625-743-01, 69625-743-02, 69625-743-03, 69625-743-04
- Packager: Cosmetique Active Production
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other information
-
Inactive ingredients
water, hydrogenated polyisobutene, glycerin, butyrospermum parkii (shea) butter, panthenol, aluminum starch octenylsuccinate, butylene glycol, propanediol, cetyl PEG/PPG-10/1 dimethicone, trihydroxystearin, zinc gluconate, madecassoside, manganese gluconate, silica, aluminum hydroxide, magnesium sulfate, disodium EDTA, copper gluconate, citric acid, acetylated glycol stearate, polyglyceryl-4 isostearate, tocopherol, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, sodium benzoate, phenoxyethanol, chlorhexidine digluconate, titanium dioxide
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA ROCHE POSAY LABORATOIRE DERMATOLOGIQUE CICAPLAST BALM B5 THERAPEUTIC MULTIPURPOSE
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69625-743 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) GLYCERIN (UNII: PDC6A3C0OX) SHEA BUTTER (UNII: K49155WL9Y) PANTHENOL (UNII: WV9CM0O67Z) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PROPANEDIOL (UNII: 5965N8W85T) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) ZINC GLUCONATE (UNII: U6WSN5SQ1Z) MADECASSOSIDE (UNII: CQ2F5O6YIY) MANGANESE GLUCONATE (UNII: 9YY2F980SV) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) EDETATE DISODIUM (UNII: 7FLD91C86K) COPPER GLUCONATE (UNII: RV823G6G67) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ACETYLATED GLYCOL STEARATE (UNII: K71M6R9N7E) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) TOCOPHEROL (UNII: R0ZB2556P8) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) SODIUM BENZOATE (UNII: OJ245FE5EU) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69625-743-01 1 in 1 CARTON 03/15/2021 1 40 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69625-743-02 1 in 1 CARTON 03/15/2021 2 3 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:69625-743-03 1 mL in 1 PACKET; Type 0: Not a Combination Product 03/15/2021 4 NDC:69625-743-04 1 in 1 CARTON 01/01/2023 4 100 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 03/15/2021 Labeler - Cosmetique Active Production (282658798) Establishment Name Address ID/FEI Business Operations Cosmetique Active Production 282658798 manufacture(69625-743) Establishment Name Address ID/FEI Business Operations L'OREAL USA PRODUCTS, INC. 624244349 pack(69625-743)