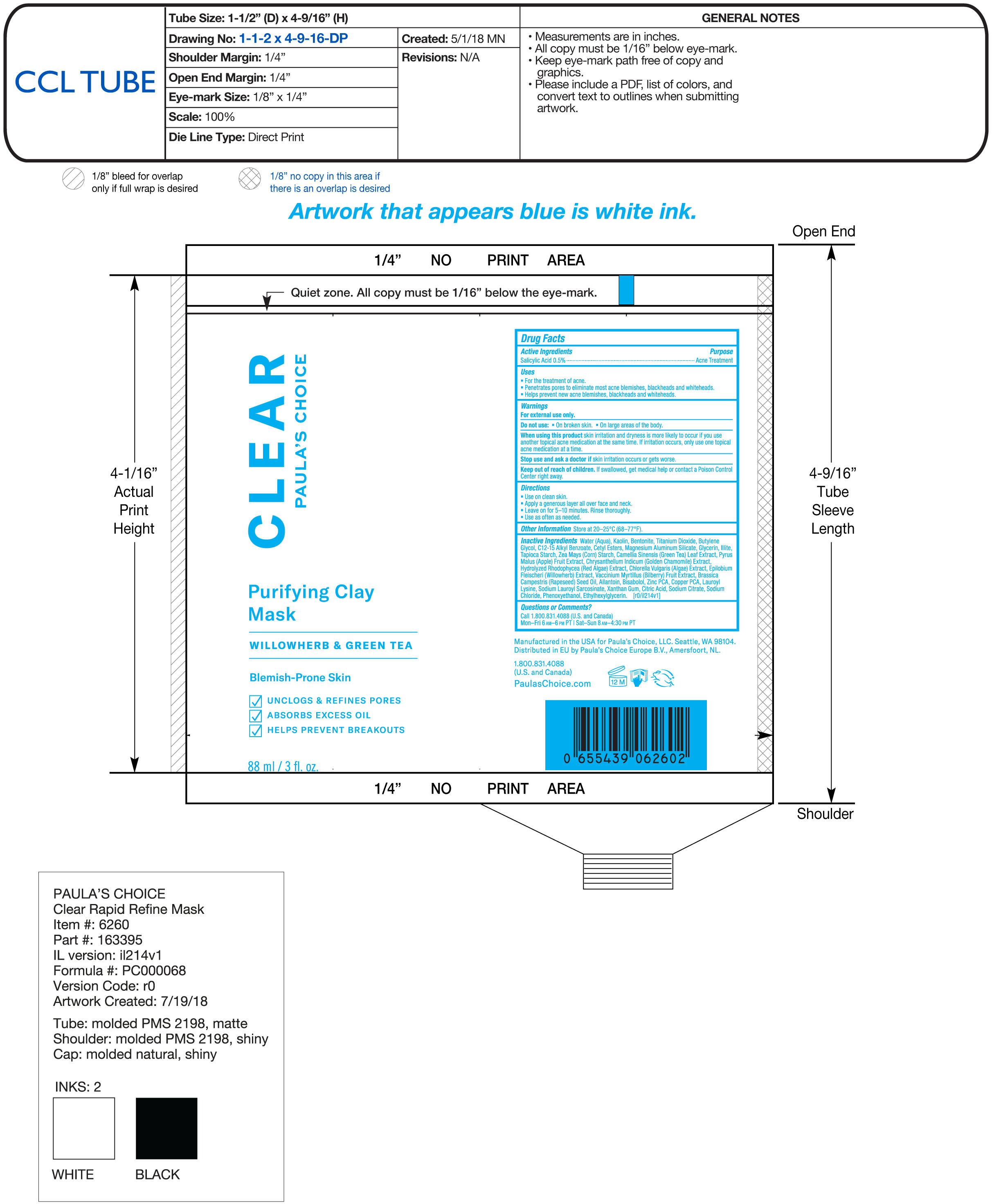
Label: PURIFYING CLAY MASK- salicylic acid cream
- NDC Code(s): 56152-5012-1
- Packager: Cosmetic Enterprises Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredientsWater (Aqua), Kaolin, Bentonite, Titanium Dioxide, Butylene Glycol, C12-15 Alkyl Benzoate, Cetyl Esters, Magnesium Aluminum Silicate, Glycerin, Illite, Tapioca Starch, Zea Mays (Corn) Starch, Camellia Sinensis (Green Tea) Leaf Extract, Pyrus Malus (Apple) Fruit Extract, Chrysanthemum Indicum (Golden Chamomile) Extract, Hydrolyzed Rhodophycea (Red Algae) Extract, Chlorella Vulgaris (Algae) Extract, Epilobium Fleischeri (Willowherb) Extract, Vaccinium Myrtillus (Bilberry) Fruit Extract, Brassica Camprestris (Rapeseed) Seed Oil, Allantoin, Bisabolol, Zinc PCA, Copper PCA, Lauroyl Lysine, Sodium Lauroyl Sarcosinate, Xanthan Gum, Citric Acid, Sodium Citrate, Sodium Chloride, Phenoxyethanol, Ethylhexylglycerin
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PURIFYING CLAY MASK
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:56152-5012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) BENTONITE (UNII: A3N5ZCN45C) CHLORELLA VULGARIS (UNII: RYQ4R60M02) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BILBERRY (UNII: 9P2U39H18W) LEVOMENOL (UNII: 24WE03BX2T) ZINC PIDOLATE (UNII: C32PQ86DH4) COPPER PIDOLATE (UNII: 497G7G1SL1) BRASSICA RAPA SUBSP. OLEIFERA OIL (UNII: N4G8379626) STARCH, CORN (UNII: O8232NY3SJ) LEDIKITE (UNII: D7BC5B0F46) CETYL ESTERS WAX (UNII: D072FFP9GU) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) GLYCERIN (UNII: PDC6A3C0OX) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) LAUROYL LYSINE (UNII: 113171Q70B) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) APPLE (UNII: B423VGH5S9) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) XANTHAN GUM (UNII: TTV12P4NEE) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALLANTOIN (UNII: 344S277G0Z) STARCH, TAPIOCA (UNII: 24SC3U704I) GREEN TEA LEAF (UNII: W2ZU1RY8B0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:56152-5012-1 88 mL in 1 TUBE; Type 0: Not a Combination Product 07/05/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/05/2018 Labeler - Cosmetic Enterprises Ltd (017701475)