Label: VISIBLE DIFFERENCE SKIN BALANCING SUNSCREEN SPF 15- octinoxate and oxybenzone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 67938-1000-1, 67938-1000-2 - Packager: Elizabeth Arden, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 1, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- INDICATIONS AND USAGE
-
WARNINGS
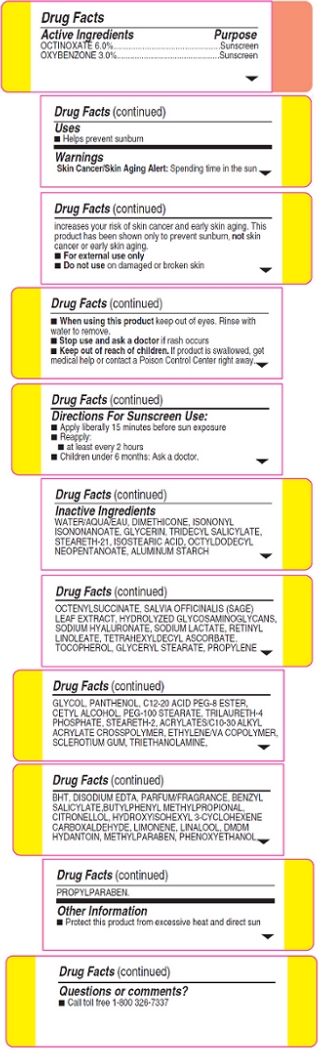
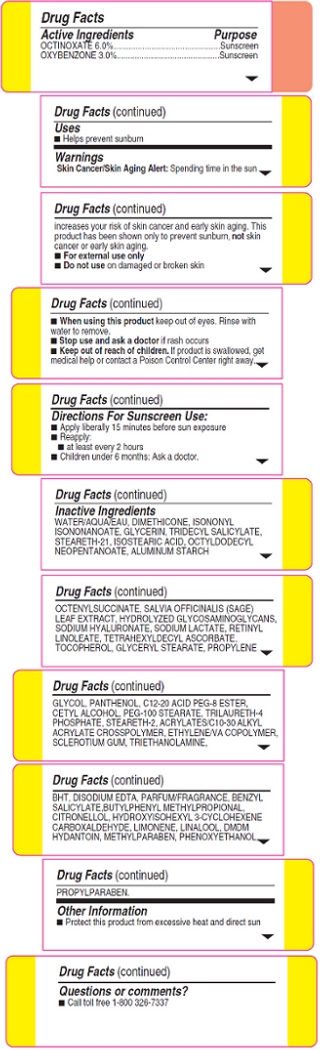
Warnings:
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
- OTC - ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive Ingredients: Water/Aqua/Eau, Dimethicone, Isononyl Isononanoate, Glycerin, Tridecyl Salicylate, Steareth-21, Isostearic Acid, Octyldodecyl Neopentanoate, Aluminum Starch Octenylsuccinate, Salvia Officinalis (Sage) Leaf Extract, Hydrolyzed Glycosaminoglycans, Sodium Hyaluronate, Sodium Lactate, Retinyl Linoleate, Tetrahexyldecyl Ascorbate, Tocopherol, Glyceryl Stearate, Propylene Glycol, Panthenol, C12-20 Acid PEG-8 Ester, Cetyl Alcohol, PEG-100 Stearate, Trilaureth-4 Phosphate, Steareth-2 Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Ethylene/VA Copolymer, Sclerotium Gum, Triethanolamine, BHT, Disodium EDTA, Parfum/Fragrance, Benxyl Salicylate, Butylphenyl Methylpropional, Citronellol, Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, Limonene, Linalool, DMDM Hydantoin, Methylparaben, Phenoxyethanol, Propylparaben.
- DOSAGE & ADMINISTRATION
- OTC - KEEP OUT OF REACH OF CHILDREN
- OTC - PURPOSE
- OTC - WHEN USING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VISIBLE DIFFERENCE SKIN BALANCING SUNSCREEN SPF 15
octinoxate and oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67938-1000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 50 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 30 mg in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) TRIDECYL SALICYLATE (UNII: AZQ08K38Z1) STEARETH-21 (UNII: 53J3F32P58) ISOSTEARIC ACID (UNII: X33R8U0062) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) SAGE (UNII: 065C5D077J) HYDROLYZED GLYCOSAMINOGLYCANS (BOVINE; 50000 MW) (UNII: 997385V0VV) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM LACTATE (UNII: TU7HW0W0QT) RETINYL LINOLEATE (UNII: 61911N8D6W) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) TOCOPHEROL (UNII: R0ZB2556P8) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PANTHENOL (UNII: WV9CM0O67Z) PEG-100 STEARATE (UNII: YD01N1999R) TRILAURETH-4 PHOSPHATE (UNII: M96W2OLL2V) STEARETH-2 (UNII: V56DFE46J5) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZYL SALICYLATE (UNII: WAO5MNK9TU) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) DMDM HYDANTOIN (UNII: BYR0546TOW) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67938-1000-1 1 in 1 CARTON 1 NDC:67938-1000-2 50 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/16/2011 Labeler - Elizabeth Arden, Inc (849222187)