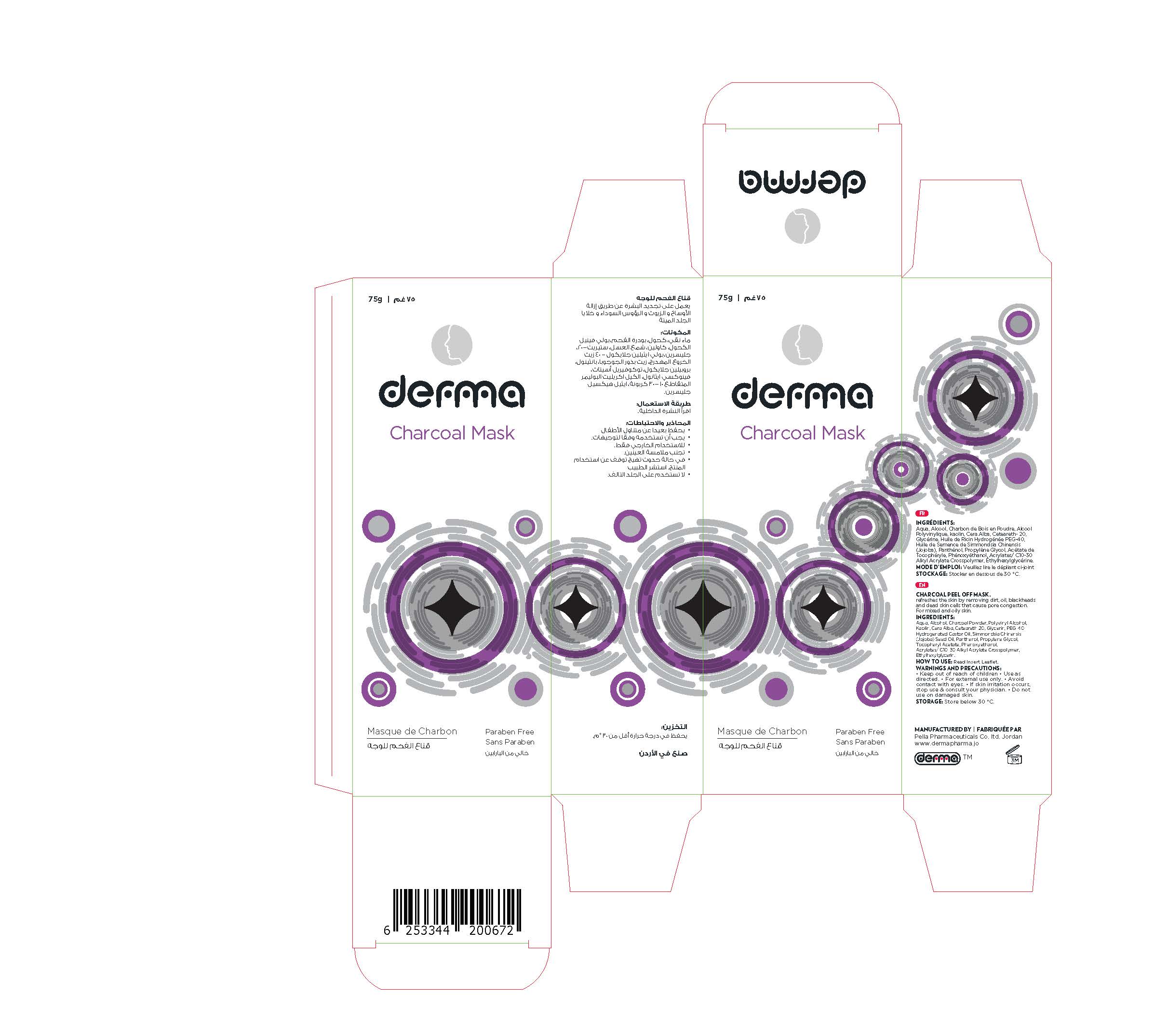
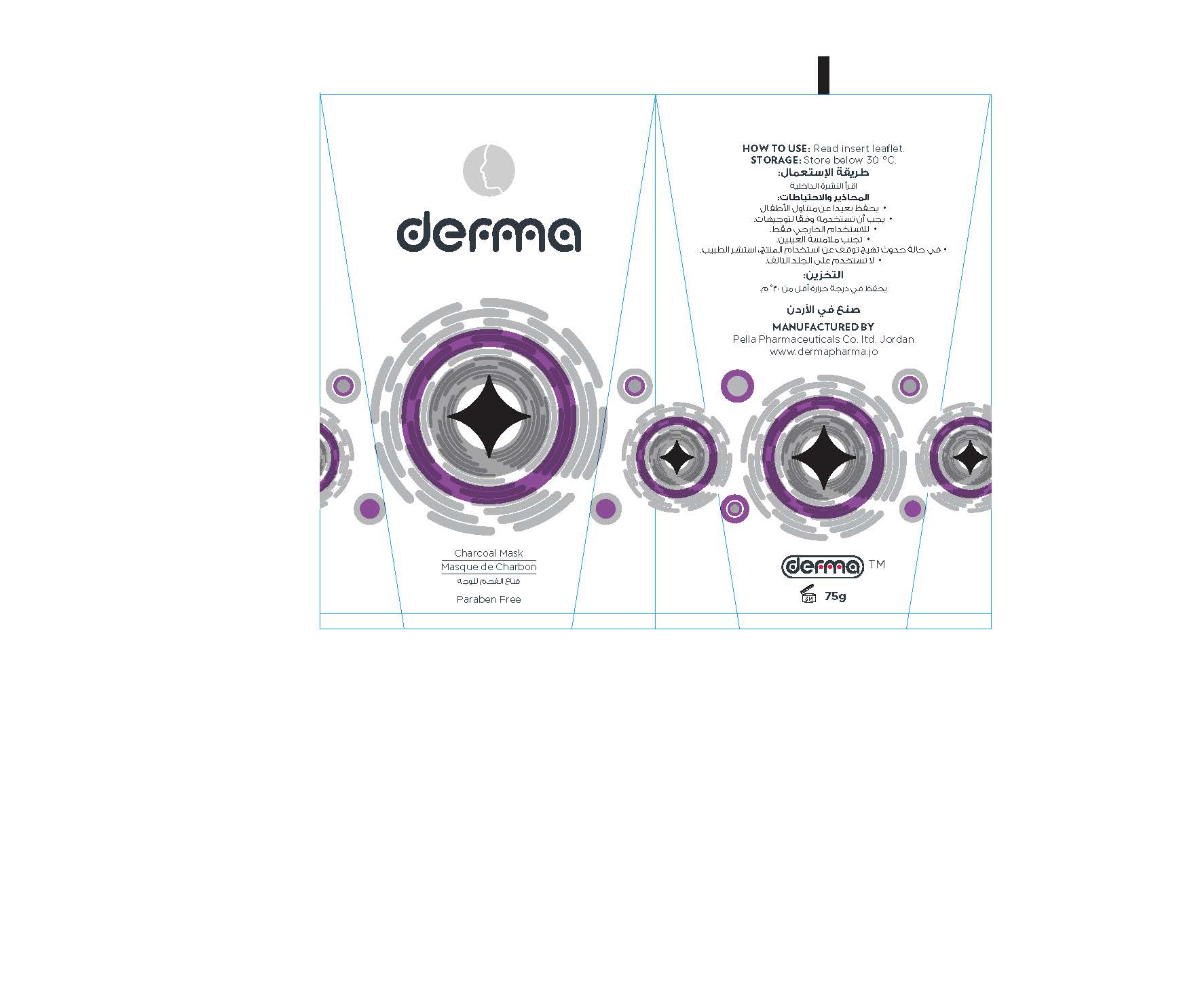
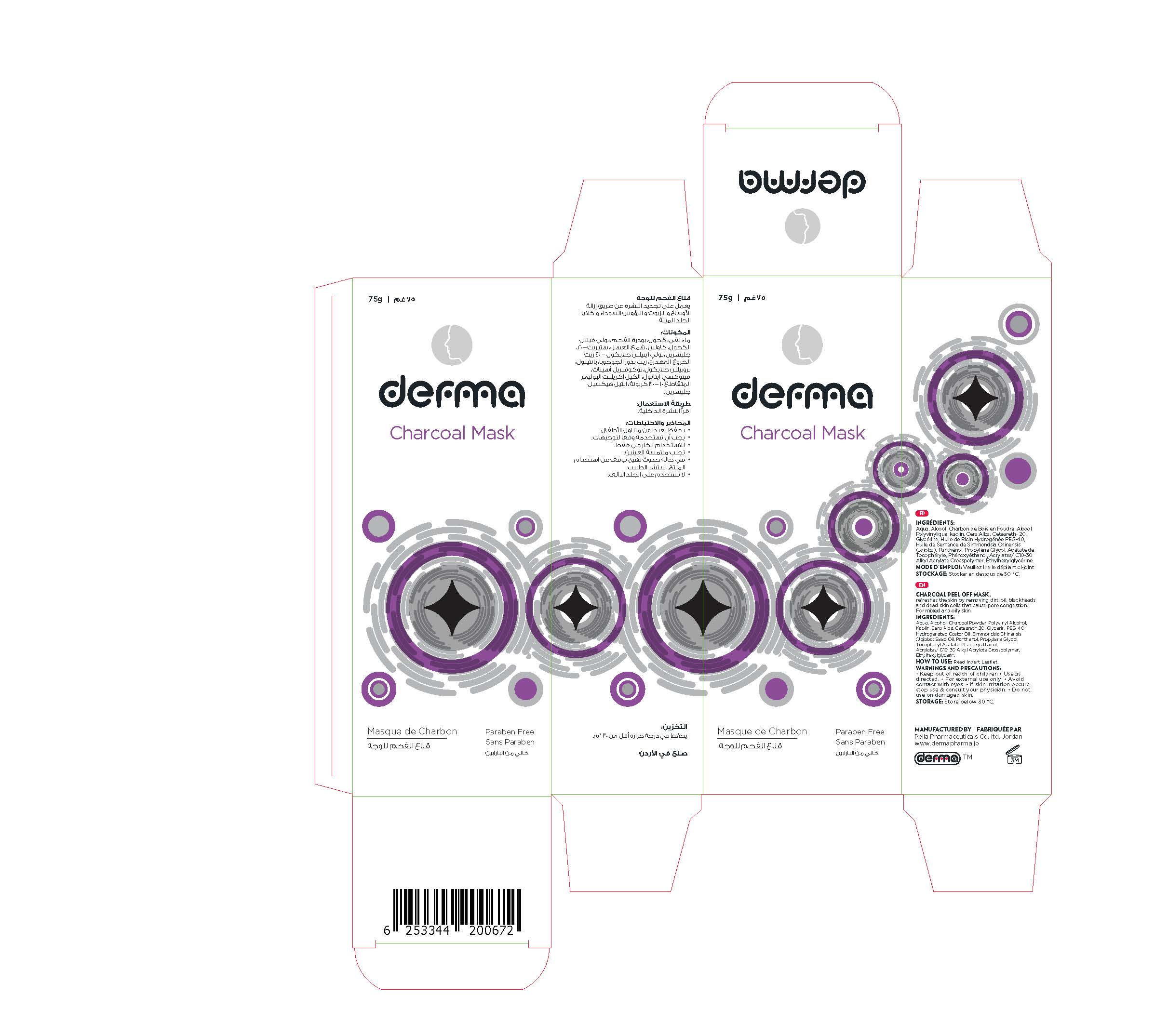
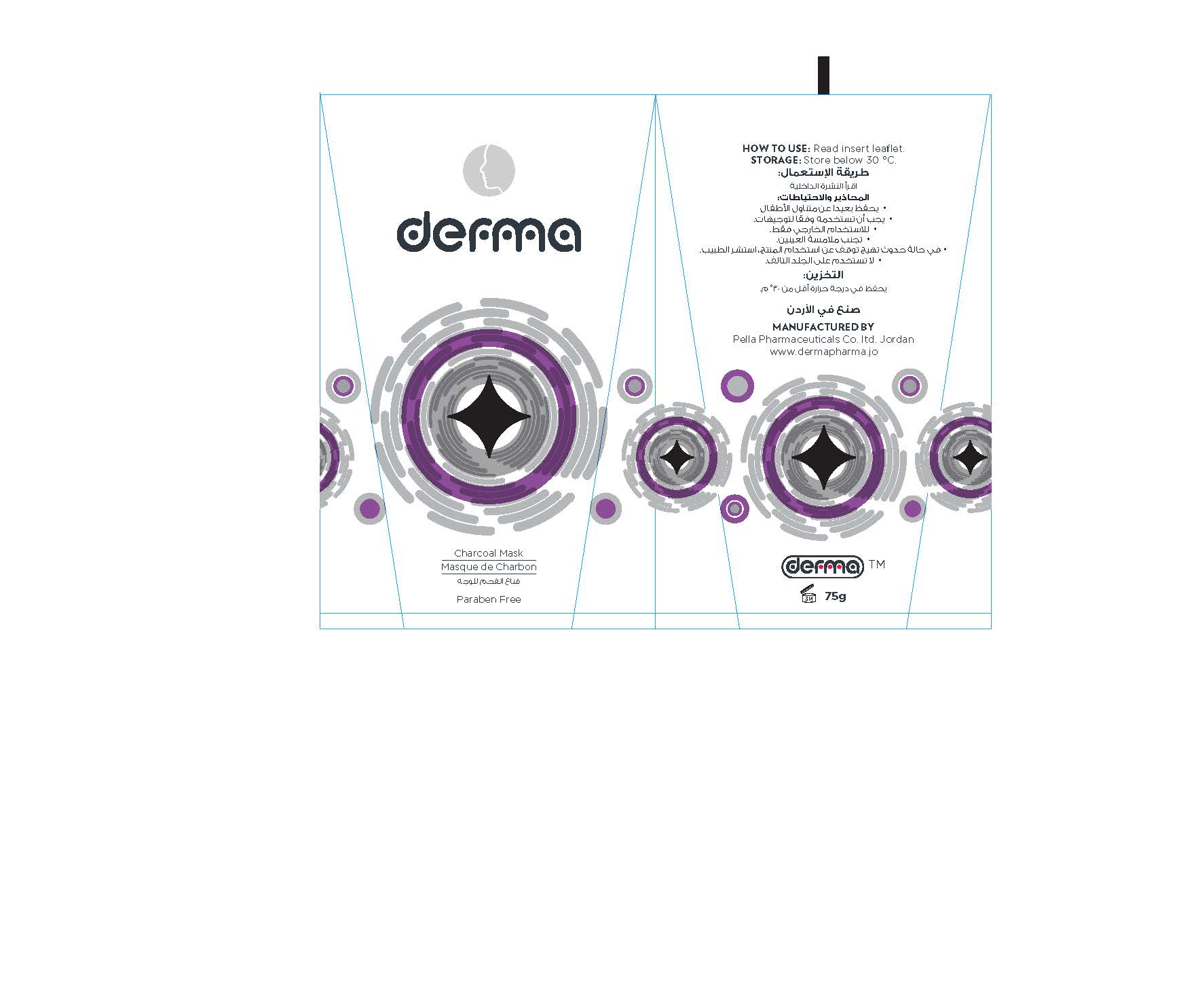
Label: DERMA CHARCOAL MASK- charcoal powder gel
- NDC Code(s): 82160-672-01
- Packager: Pella Pharmaceuticals Co .Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 21, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Forms and Presentation
- Active Ingredients
- Inactive ingredients
-
Properties
Charcoal face Mask is an advanced and powerful formula firms and refreshes the skin by removing dirt, oil, blackheads and dead skin cells that cause pore congestion. Draws toxins, chemicals, and other micro-particles to the surface of the skin.
Charcoal Face Mask is recommended for acne-prone, combination, normal and oily skin.Perfume Free, Paraben Free
- Purpose
- Indication
- Contraindication
- Precautions
- Warnings
- Pregnancy
- Lactation
- Interactions
- Side effects
-
Dosage and administration
1. Clean the face; open your pores using a warm, moist towel on face for 3- 5 minutes.
2. Gently apply mask on the face using the applicator once water is dry. Apply generously on nose, chin, forehead and cheeks. Avoid contact with your eyes, eye-brows and lip around.
3. Allow the mask to set on your skin for 20 - 30 minutes, and then peel off the mask starting from the bottom and moving up slowly when it is become dry.4. Rinse face with warm water to remove remaining bits of mask.
5. Recommended usage is once a week.
- Storage Condition
- Secondary Package
- Primary Package
-
INGREDIENTS AND APPEARANCE
DERMA CHARCOAL MASK
charcoal powder gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82160-672 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 75 mg in 75 g JOJOBA OIL (UNII: 724GKU717M) (JOJOBA OIL - UNII:724GKU717M) JOJOBA OIL 15 mg in 75 g DEXPANTHENOL (UNII: 1O6C93RI7Z) (DEXPANTHENOL - UNII:1O6C93RI7Z) DEXPANTHENOL 15 mg in 75 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) WATER (UNII: 059QF0KO0R) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) ALCOHOL (UNII: 3K9958V90M) KAOLIN (UNII: 24H4NWX5CO) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WHITE WAX (UNII: 7G1J5DA97F) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color black Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82160-672-01 1 in 1 CARTON 12/03/2020 1 75 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/03/2020 Labeler - Pella Pharmaceuticals Co .Ltd (562370925)