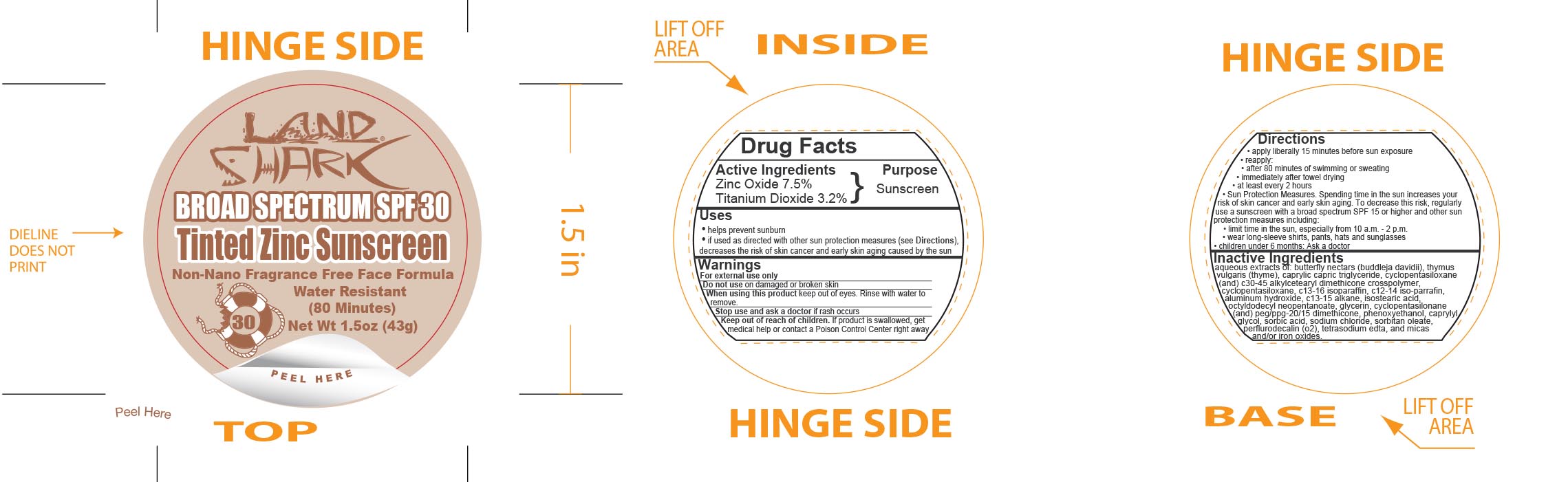
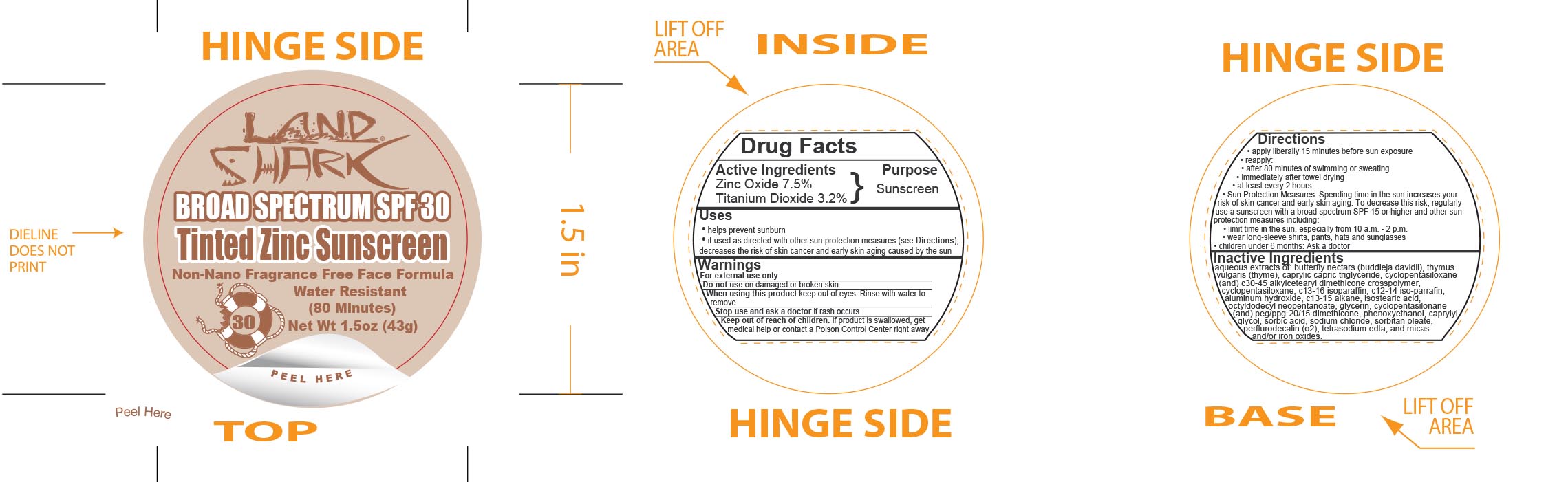
Label: LAND SHARK SPF 30 TINTED ZINC- zinc oxide and titanium dioxide cream
- NDC Code(s): 52854-944-01
- Packager: Tropical Seas, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
aqueous extracts of: butterfly nectars (buddleja davidii), thymus vulgaris (thyme), caprylic capric triglyceride, cyclopentasiloxane (and) c30-45 alkylcetearyl dimethicone crosspolymer, c13-16 isoparaffin, c12-14 iso-parrafin, aluminum hydroxide, c13-15 alkane, isostearic acid, octyldodecyl neopentanoate, glycerin, cyclopentasilonane (and) peg/ppg-20/15 dimethicone, phenoxyethanol, caprylyl glycol, sorbic acid, sodium chloride, sorbitan leate, perflurodecalin (o2), tetrasodium edta, and micas and/or iron oxides.
- Other Information
- Questions or comments?
- Principal Display Panel - 43 g Jar Label
-
INGREDIENTS AND APPEARANCE
LAND SHARK SPF 30 TINTED ZINC
zinc oxide and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52854-944 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 7.5 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 3.2 g in 100 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) WATER (UNII: 059QF0KO0R) BUDDLEJA DAVIDII LEAF (UNII: X380815D32) THYMUS VULGARIS LEAF (UNII: GRX3499643) CAPRYLIC/CAPRIC ACID (UNII: DI775RT244) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) C13-16 ISOPARAFFIN (UNII: LED42LZG6O) C12-14 ISOPARAFFIN (UNII: WP37Z9V66A) C13-15 ALKANE (UNII: 114P5I43UJ) ISOSTEARIC ACID (UNII: X33R8U0062) PEG/PPG-20/15 DIMETHICONE (UNII: 06R6X77P9C) BIS-PHENYLPROPYL DIMETHICONE (15 CST) (UNII: 4836494CFT) GLYCERIN (UNII: PDC6A3C0OX) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) PERFLUOROMETHYLDECALIN (UNII: VWJ68OB6TM) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBIC ACID (UNII: X045WJ989B) SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) DITETRACYCLINE TETRASODIUM EDETATE (UNII: WX0A0IT7K5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52854-944-01 43 g in 1 JAR; Type 0: Not a Combination Product 10/18/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/18/2021 Labeler - Tropical Seas, Inc. (627865660) Establishment Name Address ID/FEI Business Operations Tropical Seas, Inc. 627865660 manufacture(52854-944)