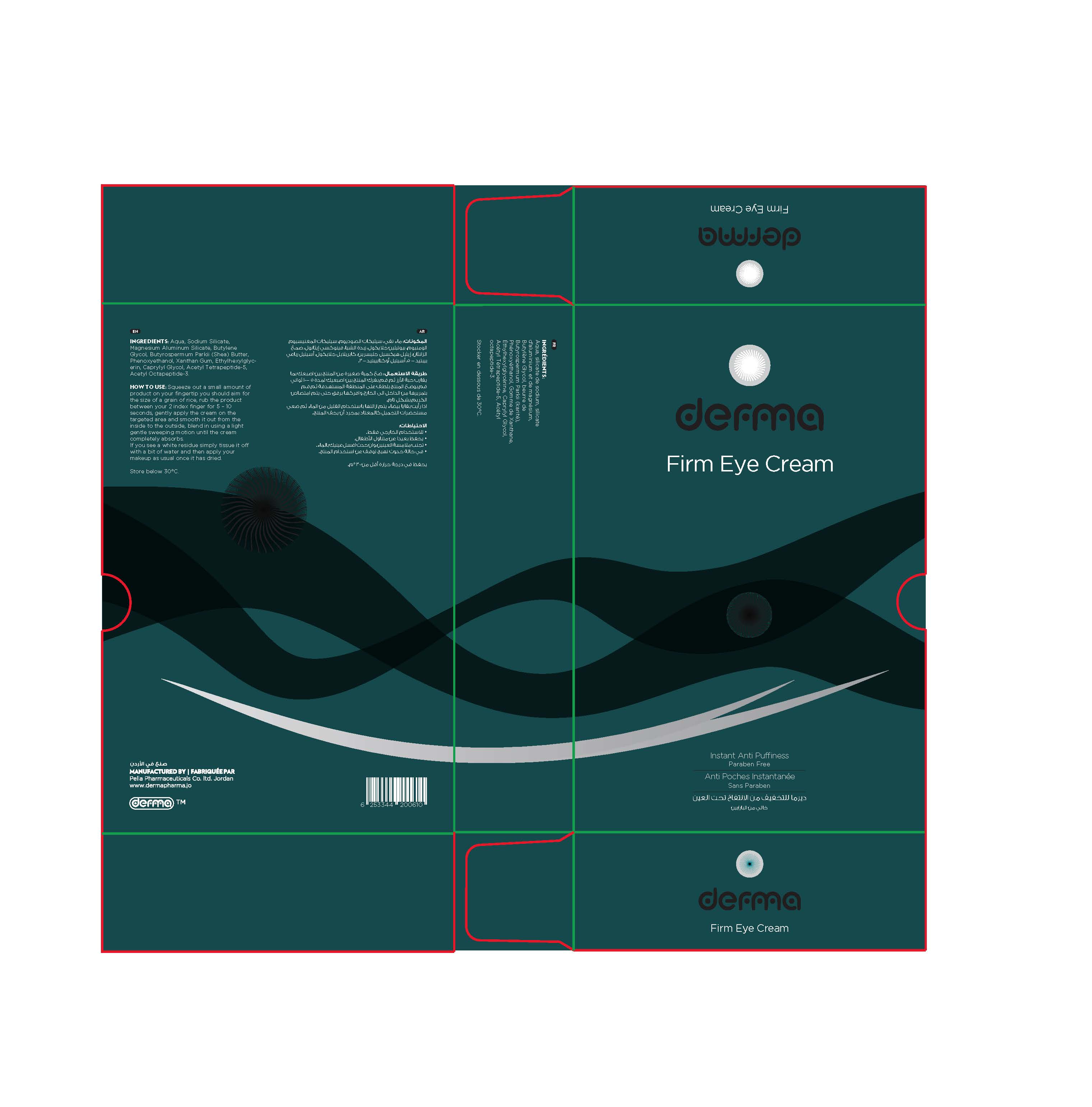
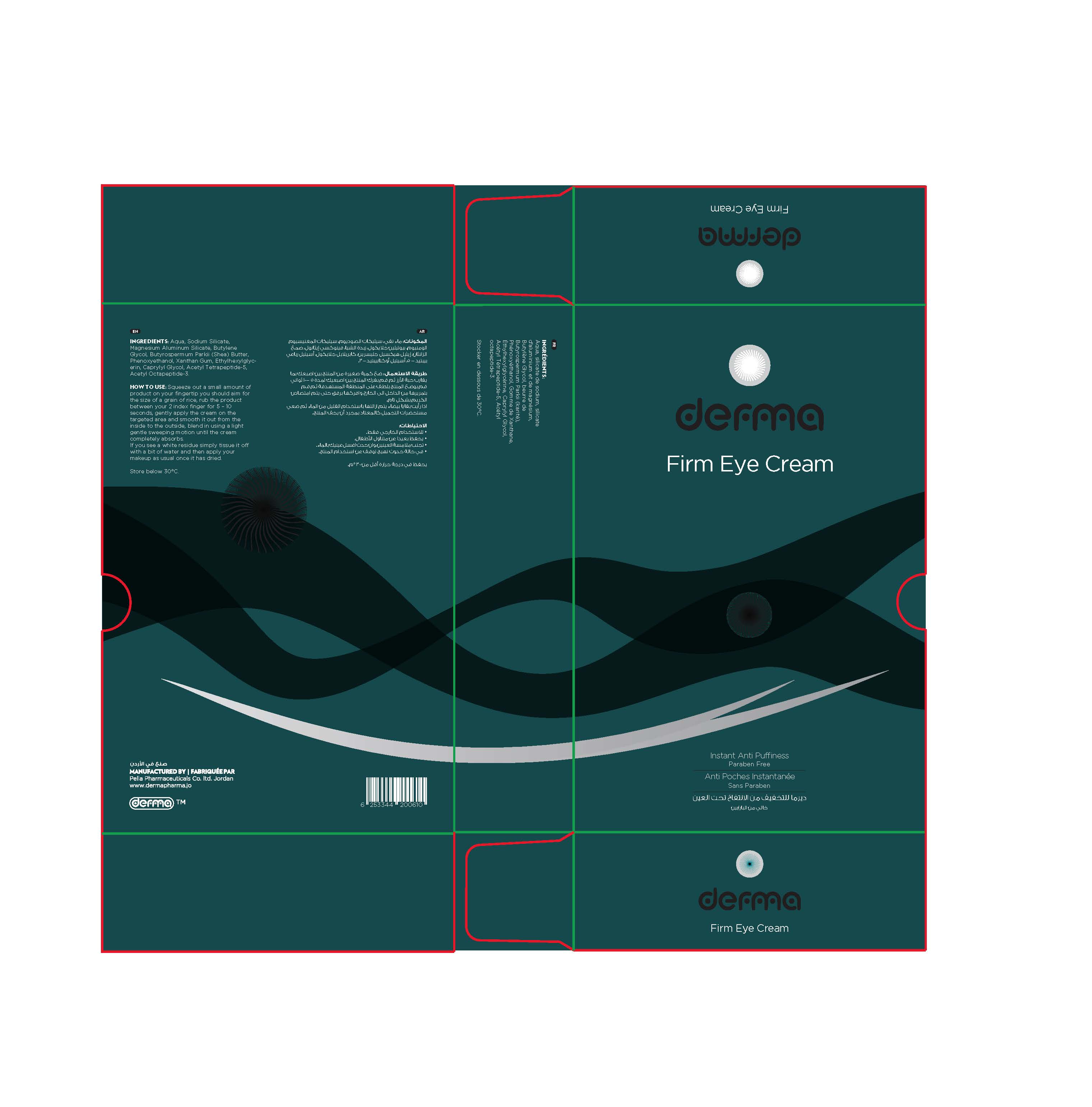
Label: DERMA FIRM EYE- acetyl tetrapeptide-5, acetyl octapeptide-3 cream
- NDC Code(s): 82160-610-01
- Packager: Pella Pharmaceuticals Co. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 18, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Form and Presentation
- Purpose
- Active Ingredients
- Composition
-
Properties
Derma firm eye is an effective Instant Anti-Puffiness which helps to diminish eye bags and the appearance of dark circles with just one single application per day.
Its revolutionary technology immediately targets eye bags and dark circles, by using a unique combination of clay minerals and a bi-peptide complex which will lead to strong skin tightening, a decrease of fluid accumulation under the eyes and a micro-muscular pause.
Perfume, dyes and paraben free.
- Indication
- Contraindicatons
-
Precautions
- Must only be used as directed.
- Avoid excessive sun exposure.
- Do not use on damaged skin (e.g. open or infected wounds, third degree burns, acne, eczema). Only use on normal healthy, non-infected skin.
- To ensure optimal performance and to avoid dependence on the product, it is advised to allow the under-eye skin to rest a few days (4-5) after using the product daily for 4 weeks. In case of occasional use of the product there is no need for a resting period.
- Do not use if the packaging has been damaged.
- Not recommended for children under the age of 16.
- Once opened, it’s advised to use the product within 3 months.
- Warnings
- Pregnancy
- Lactation
- Interactions
- Side effects
-
Dosage and administration
1. Wash your face gently and pat dry.
2. Apply an eye moisturizer (non-oil based) and wait for a minute or two so it can be completely absorbed into the skin.
3. Squeeze out a small amount of product on your fingertip. You should aim for the size of a grain of rice; any less will be not enough, and any more is generally too much and won’t be fully absorbed into the skin or could cause sensitivity.
4. Rub the product between your 2 index fingers for 5 – 10 seconds. Make sure there is still enough cream between the fingers. In case it doesn’t feel wet anymore, take a small amount on your fingertip again.
5. Gently apply the cream on your entire under eye area, and smooth it out from the inside to the outside with one finger. Blend in using a light gentle sweeping motion until the cream completely absorbs into the skin.
6. Remain expressionless for 2-3 minutes while the product dries to achieve dramatic results (fanning will accelerate the process). Simply tissue off the excess of cream with a bit of water if too much cream is applied. Wash hands and close the tube after use.To Remove: Cleanse applied area with water and pat dry.
For under make up:
First, apply your usual products (eye serum, daily moisturizer, etc.) and allow time for these products to penetrate the layers of your skin (a minute or two). Then you can apply a very thin layer of derma firm eye to the targeted area as instructed.
Once the product has dried, apply your makeup as usual.
If you see a white residue you have either used too much product or did not use moisturizer. Simply tissue off the white residue with a bit of water.
- Only use once a day. Do not use the product continuously for more than 4 weeks.
- The effects will last anywhere from 8 – 10 hours on average, without makeup the effects can last longer.
- Storage Condition
- Primary Package
- Secondary Package
-
INGREDIENTS AND APPEARANCE
DERMA FIRM EYE
acetyl tetrapeptide-5, acetyl octapeptide-3 creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82160-610 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) (ACETYL TETRAPEPTIDE-5 - UNII:Y1DFQ308G8) ACETYL TETRAPEPTIDE-5 0.0075 mg in 15 g ACETYL OCTAPEPTIDE-3 (UNII: 8K14HJF88S) (ACETYL OCTAPEPTIDE-3 - UNII:8K14HJF88S) ACETYL OCTAPEPTIDE-3 0.00375 mg in 15 g Inactive Ingredients Ingredient Name Strength XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM SILICATE (UNII: IJF18F77L3) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82160-610-01 1 in 1 CARTON 09/13/2020 1 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/13/2020 Labeler - Pella Pharmaceuticals Co. Ltd (562370925)