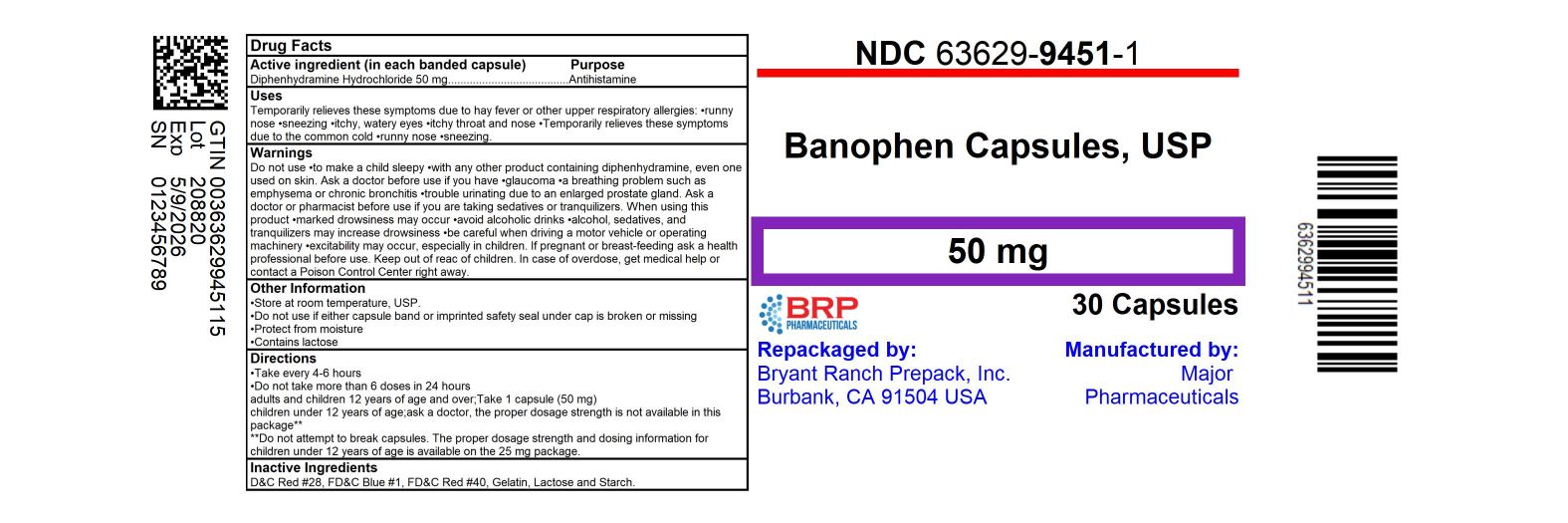
Label: BANOPHEN- diphenhydramine hcl capsule
- NDC Code(s): 63629-9451-1, 63629-9451-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0904-5307
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each banded capsule)
- Purpose
- Use
-
WARNINGS
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
-
Directions
- Take every 4-6 hours
- Do not take more than 6 doses in 24 hours
adults and children 12 years of age and over Take 1 capsule (50 mg) children under 12 years of age ask a doctor, the proper dosage strength is not available in this package** **Do not attempt to break capsules. The proper dosage strength and dosing information for children under 12 years of age is available on the 25 mg package. - Other Information
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BANOPHEN
diphenhydramine hcl capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63629-9451(NDC:0904-5307) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color pink Score no score Shape CAPSULE Size 14mm Flavor Imprint Code CPC;836 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-9451-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2022 2 NDC:63629-9451-2 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/02/2009 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-9451) , RELABEL(63629-9451)