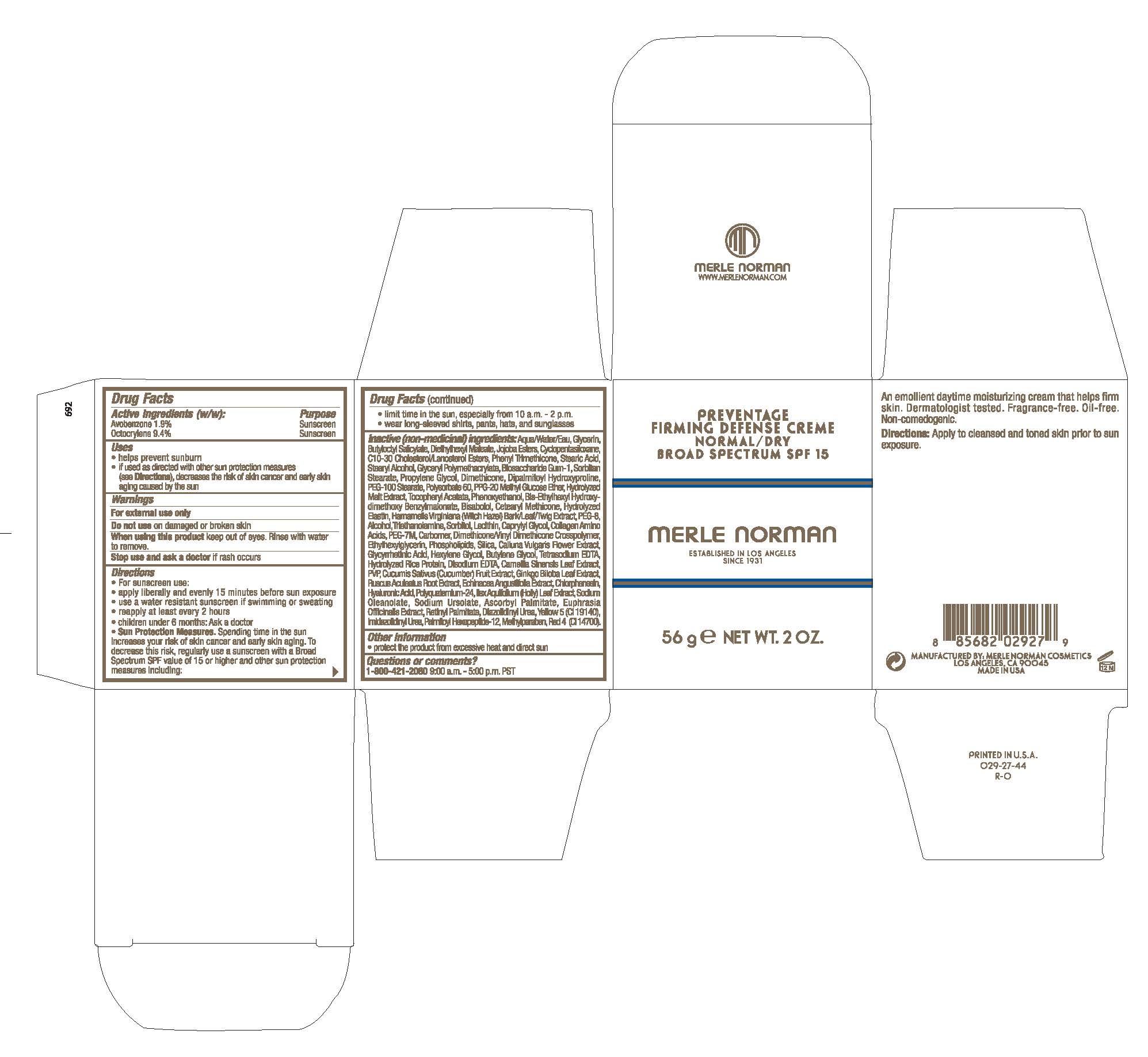
Label: PREVENTAGE FIRMING DEFENSE CREME NORMAL/DRY BROAD SPECTRUM SPF 15- avobenzone, octocrylene cream
- NDC Code(s): 57627-203-01
- Packager: Merle Norman Cosmetics, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 15, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Directions
For sunscreen use:
apply liberally and evenly 15 minutes before sun exposure
use a water resistant sunscreen if swimming or sweating
reapply at least every 2 hours
children under 6 months: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m. - 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
Apply to cleansed and toned skin prior to sun exposure - WARNINGS
-
INACTIVE INGREDIENT
Inactive (non-medicinal) ingredients: Aqua/Water/Eau, Glycerin, Butyloctyl Salicylate, Diethylhexyl Maleate, Jojoba Esters, Cyclopentasiloxane, C10-30 Cholesterol/Lanosterol Esters, Phenyl Trimethicone, Stearic Acid, Stearyl Alcohol, Glyceryl Polymethacrylate, Biosaccharide Gum-1, Sorbitan Stearate, Propylene Glycol, Dimethicone, Dipalmitoyl Hydroxyproline, PEG-100 Stearate, Polysorbate 60, PPG-20 Methyl Glucose Ether, Hydrolyzed Malt Extract, Tocopheryl Acetate, Phenoxyethanol, Bis-Ethylhexyl Hydroxydimethoxy Benzylmalonate, Bisabolol, Cetearyl Methicone, Hydrolyzed Elastin, Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract, PEG-8, Alcohol, Triethanolamine, Sorbital, Lecithin, Caprylyl Glycol, Collagen Amino Acids, PEG-7M, Carbomer, Dimethicone/Vinyl Dimethicone Crosspolymer, Ethylhexylglycerin, Phospholipids, Silica, Calluna Vulgaris Flower Extract, Glycyrrhethic Acid, Glycyrrhetinic Acid, Hexylene Glycol, Butylene Glycol, Tetrasodium EDTA, Hydrolyzed Rice Protein, Disodium EDTA, Camellia Sinensis Leaf Extract, PVP, Cucumis Sativus (Cucumber) Fruit Extract, Ginkgo Biloba Leaf Extract, Ruscus Aculeatus Root Extract, Echinacea angustifolia extract, Chlorphenesin, Hyaluronic Acid, Polyquaternium-24, Ilex Aquifolium (Holly) Leaf Extract, Sodium Oleanolate, Sodium Ursolate, Ascorbyl Palmitate, Euphrasia Officinalis Extract, Retinyl Palmitate, Diazolidinyl Urea, Yellow 5 (CI 19140), Imidazolidinyl Urea, Palmitoyl Hexapeptide-12, Methylparaben, Red 4 (CI 14700)
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREVENTAGE FIRMING DEFENSE CREME NORMAL/DRY BROAD SPECTRUM SPF 15
avobenzone, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57627-203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.9 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9.4 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM URSOLATE (UNII: NV5D25VL1O) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) CUCUMBER (UNII: YY7C30VXJT) TROLAMINE (UNII: 9O3K93S3TK) GINKGO (UNII: 19FUJ2C58T) ENOXOLONE (UNII: P540XA09DR) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GREEN TEA LEAF (UNII: W2ZU1RY8B0) AMINO ACIDS (UNII: 0O72R8RF8A) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PHENOXYETHANOL (UNII: HIE492ZZ3T) POVIDONE (UNII: FZ989GH94E) HYALURONIC ACID (UNII: S270N0TRQY) SODIUM OLEANOLATE (UNII: LVB19G8AUT) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) FD&C RED NO. 4 (UNII: X3W0AM1JLX) WATER (UNII: 059QF0KO0R) DIETHYLHEXYL MALEATE (UNII: C2F7JHI12L) C10-30 CHOLESTEROL/LANOSTEROL ESTERS (UNII: 137SL7IL0Y) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) POLYSORBATE 60 (UNII: CAL22UVI4M) HYDROLYZED BOVINE ELASTIN (BASE; 1000 MW) (UNII: ZR28QKN0WT) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) PPG-20 METHYL GLUCOSE ETHER (UNII: 3WV1T97D3K) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LEVOMENOL (UNII: 24WE03BX2T) CETEARYL METHICONE (15000 MW) (UNII: VY9RTR7MSY) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) PEG-100 STEARATE (UNII: YD01N1999R) CALLUNA VULGARIS FLOWERING TOP (UNII: D9PC510CQV) EDETATE SODIUM (UNII: MP1J8420LU) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) BASIC YELLOW 5 (UNII: 07BP340B4T) IMIDUREA (UNII: M629807ATL) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) POLYETHYLENE OXIDE 300000 (UNII: 4QIB4U4CQR) EUPHRASIA STRICTA (UNII: C9642I91WL) PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) METHYLPARABEN (UNII: A2I8C7HI9T) ILEX AQUIFOLIUM LEAF (UNII: 9Z32IEA9F7) ASCORBYL PALMITATE (UNII: QN83US2B0N) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) ALCOHOL (UNII: 3K9958V90M) RUSCUS ACULEATUS ROOT (UNII: ZW12V95I1Q) ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57627-203-01 56 g in 1 JAR; Type 0: Not a Combination Product 11/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 11/15/2021 Labeler - Merle Norman Cosmetics, Inc. (008479388) Establishment Name Address ID/FEI Business Operations Merle Norman Cosmetics, Inc. 008479388 manufacture(57627-203)