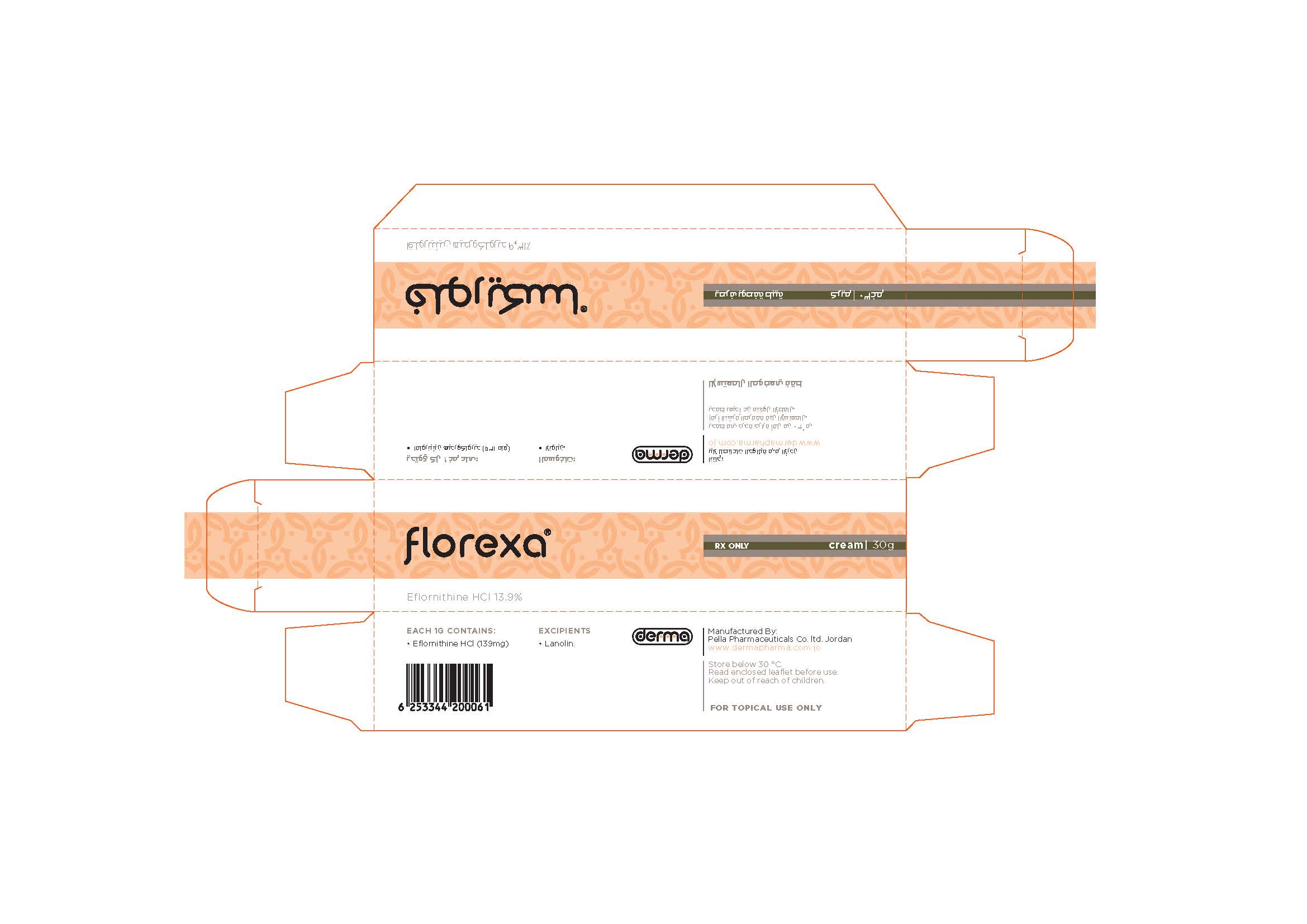
Label: FLOREXA- eflornithine hydrochloride cream
- NDC Code(s): 82160-125-01
- Packager: Pella Pharmaceuticals Co. Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Composition
-
Properties
Florexa is a prescribed medication applied to the skin for the reduction of unwanted facial hair in women.
There are no studies examining the inhibition of the enzyme ornithine decarboxylase (ODC) in human skin following the application of topical eflornithine. However, there are studies in the literature that report the inhibition of ODC activity in skin following oral eflornithine. It is postulated that topical eflornithine hydrochloride irreversibly inhibits skin ODC activity. This enzyme is necessary in the synthesis of polyamines. Animal data indicate that inhibition of ornithine decarboxylase inhibits cell division and synthetic functions, which affect the rate of hair growth. Eflornithine hydrochloride Cream 13.9% has been shown to retard the rate of hair growth in non-clinical and clinical studies. - Indications
- Contraindications
-
Precautions
For external use only.
Transient stinging or burning may occur when applied to abraded or broken skin.Pregnancy
Because there are no adequate and well-controlled studies in pregnant women, the risk / benefit ratio of using eflornithine HCl in women with unwanted facial hair who are pregnant should be weighed carefully with serious consideration for either not implementing or discontinuing use of Florexa.
- Drug Interactions
- Warnings
-
Dosage and Administration
Apply a thin layer of Florexa, to wanted areas of the face and adjacent involved areas under the chin and rub in thoroughly. Do not wash treated area for at least 4 hours. Use twice daily at least 8 hours apart or as directed by a physician. The patient should continue to use hair removal techniques as needed in conjunction with Florexa. ( Florexa should be applied at least 5 minutes after hair removal.) Cosmetics or sunscreens may be applied over treated areas; you should wait a few minutes to allow the treatment to be absorbed.
Florexa doesn't permanently remove hair or "cure" unwanted facial hair. It is not a depilatory. Your treatment program should include continuation of any hair removal technique you are currently using. Florexa will help you manage your condition and improve your appearance.Improvement in the condition occurs gradually. Don't be discouraged if you see no immediate improvement. Be patient. Improvement may be seen as early as 4 to 8 weeks of treatment. Improvement may take longer in some individuals. If no improvement is seen after 6 months of use; discontinue use.
- Overdosage
-
Side Effects
The following side effects have been reported
Acne, Pseudofolliculitis Barbae, Stinging Skin, Headache, Burning Skin, Dry Skin, Pruritus (itching), Erythema (redness), Tingling Skin, Dyspepsia, skin irritation, rash, alopecia, dizziness, folliculitis, hair ingrown, facial edema, anorexia, nausea, asthenia, vertigo. - Storage
- How supplied
-
THIS IS A MEDICAMENT
- Medicament is a product which affects your health and its consumption contrary to instructions is dangerous for you.
- Strictly follow the doctor's prescription, the method of use and the instruction of the pharmacist who sold the medicament.
- The doctor and the pharmacist are experts in medicine, its benefits and risks.
- Do not by yourself interrupt the period of treatment prescribed for you.
Do not repeat the same prescription without consulting your doctor.
- Keep medicament out of reach of children.
- Primary Package
- Secondary Package
-
INGREDIENTS AND APPEARANCE
FLOREXA
eflornithine hydrochloride creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82160-125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EFLORNITHINE HYDROCHLORIDE (UNII: 4NH22NDW9H) (EFLORNITHINE - UNII:ZQN1G5V6SR) EFLORNITHINE HYDROCHLORIDE ANHYDROUS 4170 mg in 30 g Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82160-125-01 1 in 1 CARTON 02/09/2012 1 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/09/2012 Labeler - Pella Pharmaceuticals Co. Ltd (562370925)