Label: TACRUS- tacrolimus ointment
- NDC Code(s): 82160-124-01, 82160-124-02
- Packager: Pella Pharmaceuticals Co. Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Composition
-
Indications
The active substance of Tacrus ®, tacrolimus monohydrate, is an immune-modulating agent.
Tacrus ® 0.03% ointment is used to treat moderate to severe atopic dermatitis (eczema) in adults who are not adequately responsive to or are intolerant of conventional therapies such as topical corticosteroids and in children (2 years of age and older) who failed to respond adequately to conventional therapies such as topical corticosteroids. Tacrus ® 0.1 % ointment is used to treat moderate to severe atopic dermatitis (eczema) in adults who are not adequately responsive to or are intolerant of conventional therapies such as topical corticosteroids.
Once moderate to severe atopic dermatitis is cleared or almost cleared after up to 6 weeks treatment of a flare, and if you are experiencing frequent flares (i.e. 4 oc more per year), it may be possible to prevent flares coming back or prolong the time you are free from flares by using Tacrus ® 0.1% ointment twice weekly.
In atopic dermatitis, an over-reaction of the skin's immune system causes skin inflammation (itchiness, redness, dryness).
Tacrus ® alters the abnormal immune response and relieves the skin inflammation and the itch. -
Contraindications
If you are allergic (hypersensitive) to tacrolimus or any of other ingredients of Tacrus or to macrolide anttbiotics (e.g. azithromycin, clarithromycin, erythromycin).
- Tacrolimus 0.03% is not approved in children younger than 2 years of age. Therefore it should not be used in this age group. Please consult your doctor.
- Tacms ® 0.1 % ointment is not approved for children younger than 16 years of age. Therefore it should not be used in this age group. Please consult your doctor.
- The effect of treatment with Tacrolimus on the developing immune system in children, especially the young, has not been established.
- The safety of using Tacrus ® for a long time is not known. A very small number of people who have used Tacrolimous ointment have had malignancies (for example skin or lymphoma) however a link to Tacrolimus ointment treatmert has not been shown.
- Avoid exposing the skin to long periods of sunlight or artificial sunlight such as tanning beds. If you spend time outdoors after applying Tacrus ® use sunscreen and wear loose fitting clothing that protects the skin from the sun. In addition, ask your doctor for advice on other appropriate sun protection methods. If you are prescribed light therapy, inform your doctor that you are using Tacrus ® as it is not recommended to use Tacrolimus and light therapy at the same time.
- If your doctor tells you to use Tacrus ® twice weekly to keep your atopic dermatitis cleared, your condition should be reviewed by your doctor at least every 12 months, even if it remains under control. In children, maintenance treatment should be suspended after 12 months, to assess whether the need for continued treatment still exists.
-
Precautions while taking Tacrus
®
Tell your doctor if you:
- Have liver failuer.
- Have any skin malignancies (tumors) or if you have a weakened immnne system (immuno-compromised) whatever the cause.
- Have inherited skin barrier disease such as Netherton's syndrome, lamellar ichthyosis (extensive scaling of the skin due to a thickening of the outer layer of the skin), or if you suffer from generalized erythroderma (inflammatory reddening and scalling of the entire skin).
- A cutaneous Graft Versus Host Disease (an immune reaction of the skin which is a common complication in patients who have undergone a bone marrowtransplant).
- Have swollen lymph nodes at initiation of treatment. If your lymph nodes become swollen during treatment with Tacrus ®, consult your doctor.
- Have infected lesions. Do not apply the ointment to infected lesions.
- Notice any
change to the appearance of your skin, please inform your physician.
-
Drug Interactions
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
You may use moisturizing creams and lotions during treatment with Tacrus® but these products should not be used within two hours of applying Tacrus ®.
The use of Tacrus ® at the same time as other preparations to be used on the skin or while taking oral corticosteroids (e.g. cortisone) or medicines which affect the immune system has not been studied.
Using Tacrus ® with food and drink
While using Tacrus ®, drinking alcohol may cause the skin or face to become flushed or red and feel hot. -
Dosage and Administration
Always use Tacrus ® exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
- Apply Tacrus ® as thin layer to affected areas of your skin.
- Tacrus ® may be used on most parts of the body, including the face and the neck and in the creases of your elbows and knees.
- Avoid using the ointment inside your nose or mouth or in your eyes. If the ointment gets on any of these areas, it should be thoroughly wiped off and/or rinsed off with water. Do not cover the skin being treated with bandages or wraps.
- Wash your hands after applying Tacrus ® unless your hands are also being treated. Before applying Tacrus ® after a bath or shower, be sure your skin is completely dry.
Children (2 years of age and older):
Apply Tacrolimus 0.03% ointment twice a day for up to three weeks, once in the morning and once in the evening. Afterwards the ointment should be used once a day on each affected region of the skin until the eczema has gone away.
Adults (16 years of agc and older):
Two strengths of Tacrolimus (0.03% and 0.1% ointment) are available for adults patients (16 years of age and older). Your doctor will decide which strength is best for you.
Usually, treatment is started with Tacrus ® 0.1% ointment twice a day, once in the morning and once in the evening, until the eczema has cleared. Depending on the response of your eczema your doctor will decide if the frequency of application can be reduced or the lower strength, Tacrolimus 0.03% ointment, can be used.
Treat each affected region of your skin until the eczema has gone away. Improvement is usually seen within one week. If you do not see any improvement after two weeks, see your doctor about other possible treatments. You may be told by your doctor to use Tacrus ® 0.1% ointment twice weekly once your atopic dermatitis has cleared or almost cleared (Tacrolimus 0.03% for children and Tacrs ® 0.1% for adults). Tacrus ® 0.1% ointment should be applied once a day twice weekly (e.g. Monday and Thursday) to areas of your body commonly affected by atopic dermatitis. There should be 2-3 days without Tacrus ® treatment between applications.If symptoms reappear you should use Tacrus ® twice daily as outlined above and arrange to see your doctor to review your treatment.
-
Possible Side Effects
Like all medicines, Tacrus ® can cause side effects, although not everybody gets them.
Very common (probably affecting more than 1 in 10): burning sensation and itching; these symptoms are usually mild to moderate and generally go away within one week of using Tacrus ®.
- Common (probably affecting up to 1 in 10): Redness, feeling of warmth, pain, increased skin sensitivity (especially to hot and cold) skin tingling, rash, local skin infection regardless of specific cause including but not limited to: inflamed or infected hair follicles,cold sores,generalized herpes simplex infections,facial flushing or skin irritation after drinking alcohol is also common.
- Uncommon (probably affecting less than 1 in 100): acne.
Following twice-weekly treatment application site infections have been reported in children and adults, impetigo, a superficial bacterial skin infection that usually produces blisters or sores on the skin,has been reported in children. Rosaceae (facial redness), Rosaceae- like dermatitis and oedema at the application site has been reported during post marketing experience.
Since commercial availability a very small number of people who have used Tacrolimus ointment have had malignancies (for example, skin and lymphoma). However a link to Tacrolimus ointment has not been confirmed or refuted on the available evidence so far.
If any of the side effects gets serious, or if you notice any side effect not listed in this leaflet, please tell your doctor or pharmacist. -
Overdosage
If you accidentaly swallow some ointment
If you accidentally swallow the ointment, consult your doctor or pharmacist as soon as possible. Do not try to induce Vomiting.
If you forget to use Tacrus ®If you forget to apply the ointment at the scheduled time, do it as soon as you remember and then continue as before. If you have any further questions on the use of this product, ask your doctor or pharmacist.
- How supplied
- Storage
-
THIS IS A MEDICAMENT
- Medicament is a product which affects your health and its consumption contrary to instructions is dangerous for you.
- Strictly follow the doctor's prescription, the method of use and the instruction of the pharmacist who sold the medicament.
- The doctor and the pharmacist are experts in medicine, its benefits and risks.
- Do not by yourself interrupt the period of treatment prescribed for you.
- Do not repeat the same prescription without consulting your doctor.
- Keep medicament out of reach of children.
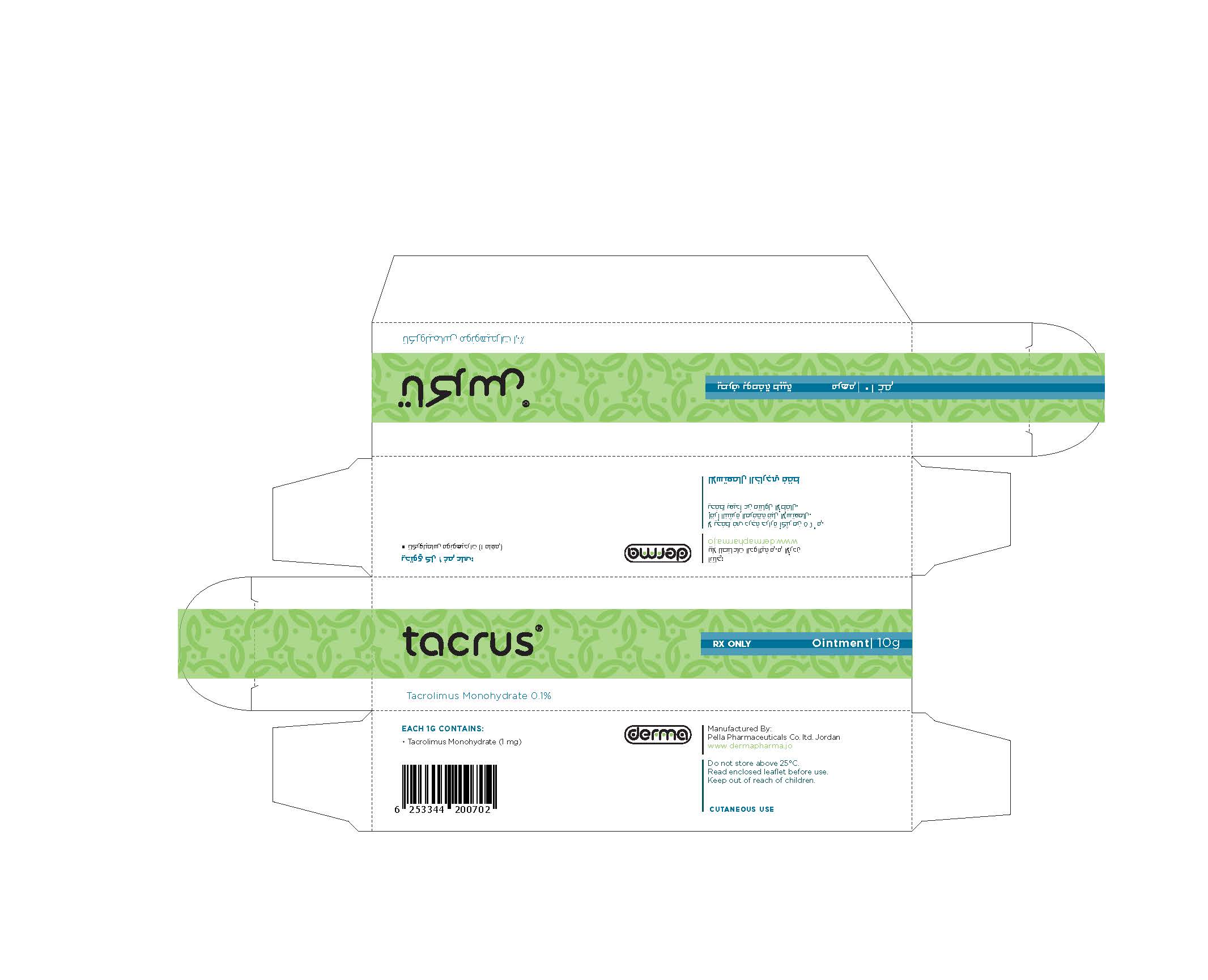
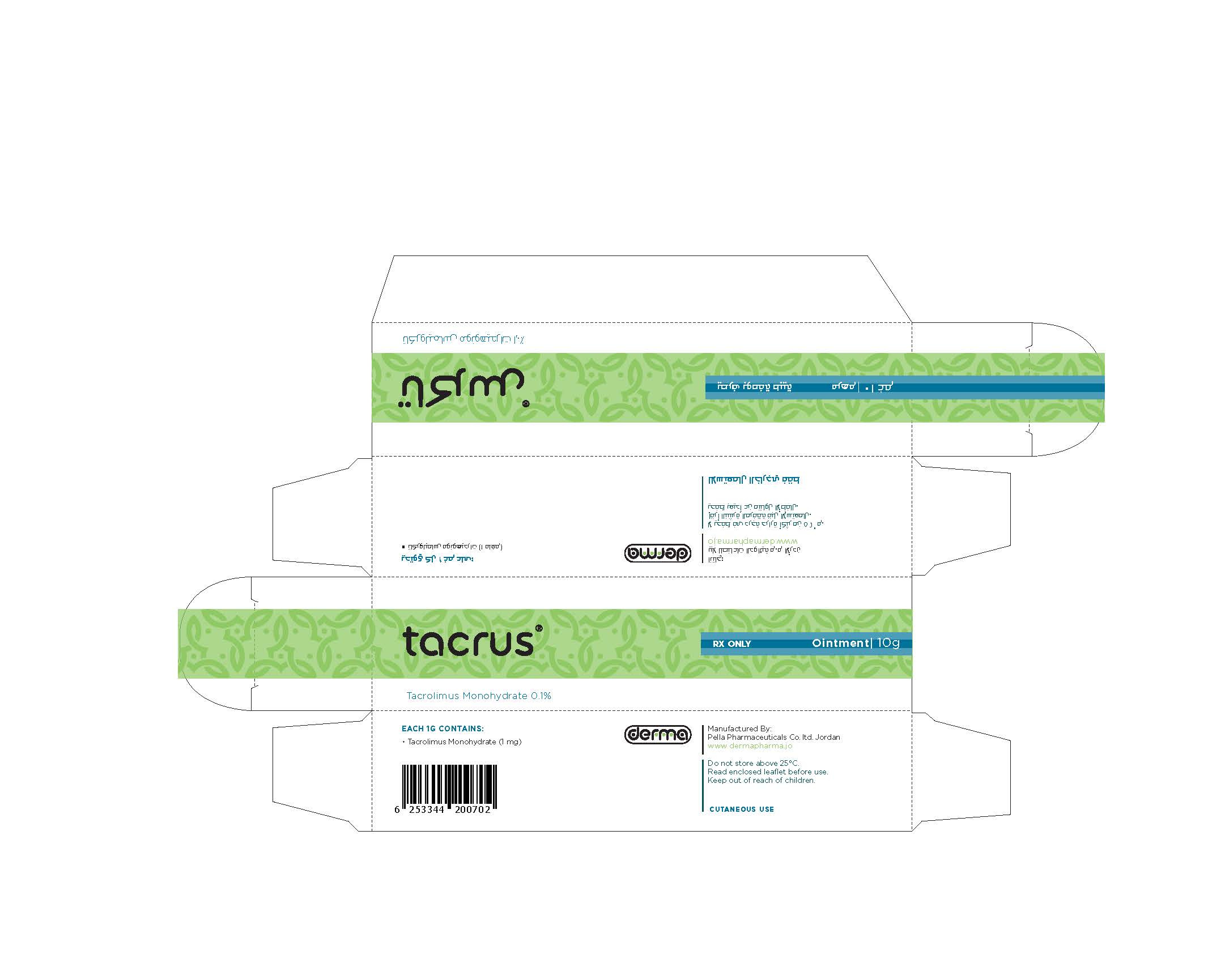
- Secondary Package 10 g
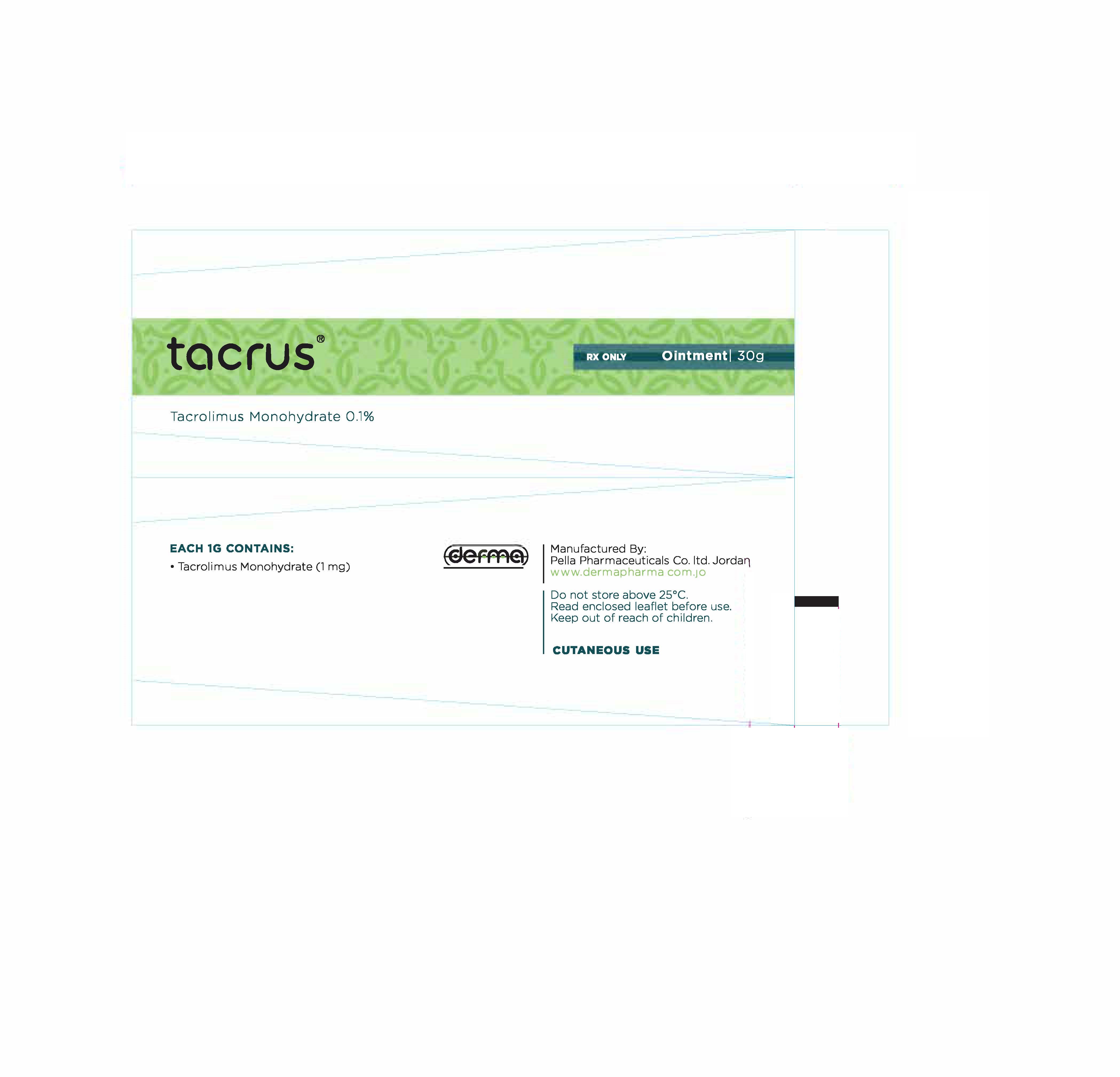
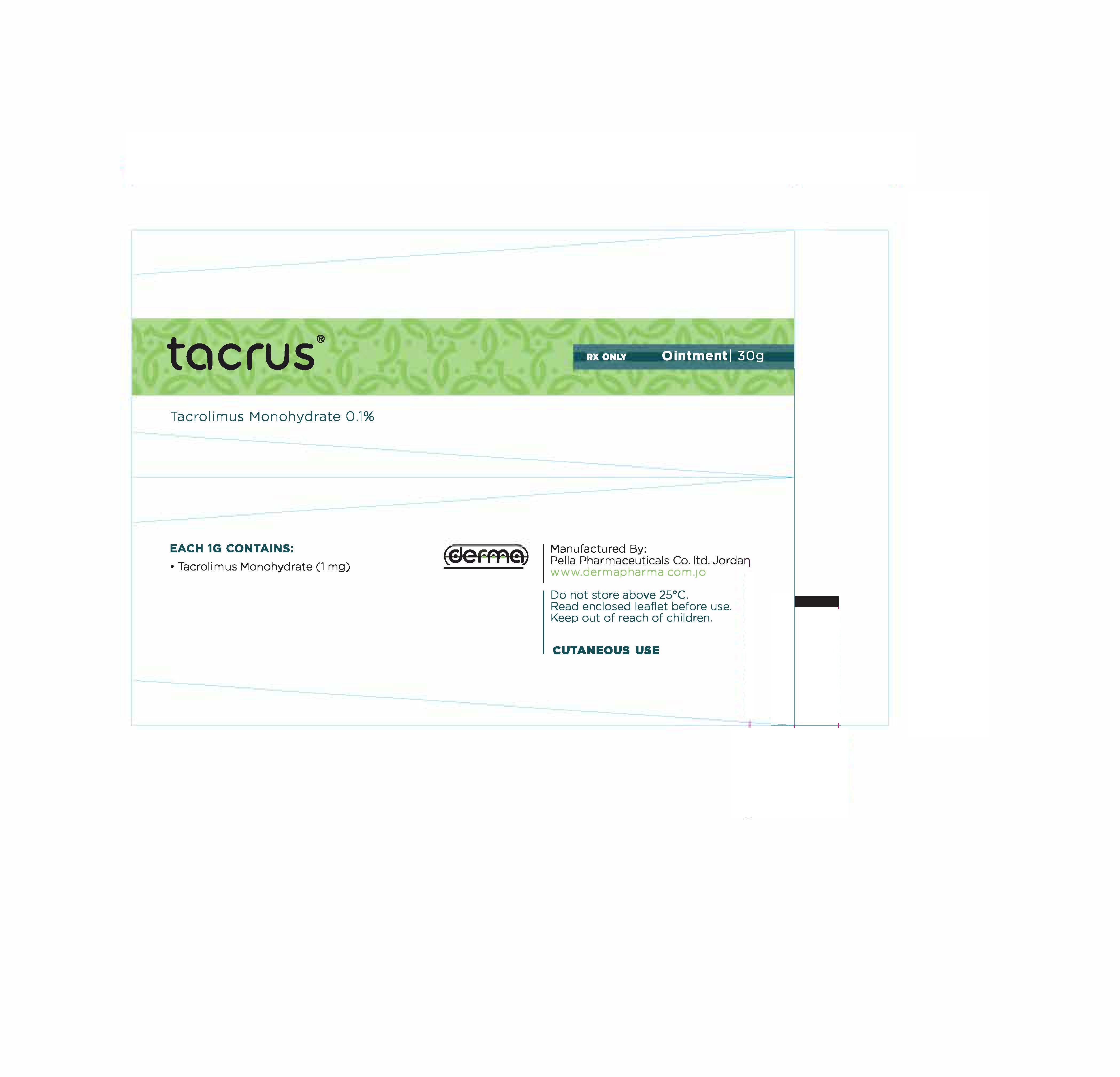
- Secondary Package 30 g
- Primary Package 30 g
- Primary Package 10 g
-
INGREDIENTS AND APPEARANCE
TACRUS
tacrolimus ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82160-124 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TACROLIMUS (UNII: WM0HAQ4WNM) (TACROLIMUS ANHYDROUS - UNII:Y5L2157C4J) TACROLIMUS ANHYDROUS 30 mg in 30 g Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82160-124-01 1 in 1 CARTON 12/11/2014 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:82160-124-02 1 in 1 CARTON 12/11/2014 2 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/11/2014 Labeler - Pella Pharmaceuticals Co. Ltd (562370925)