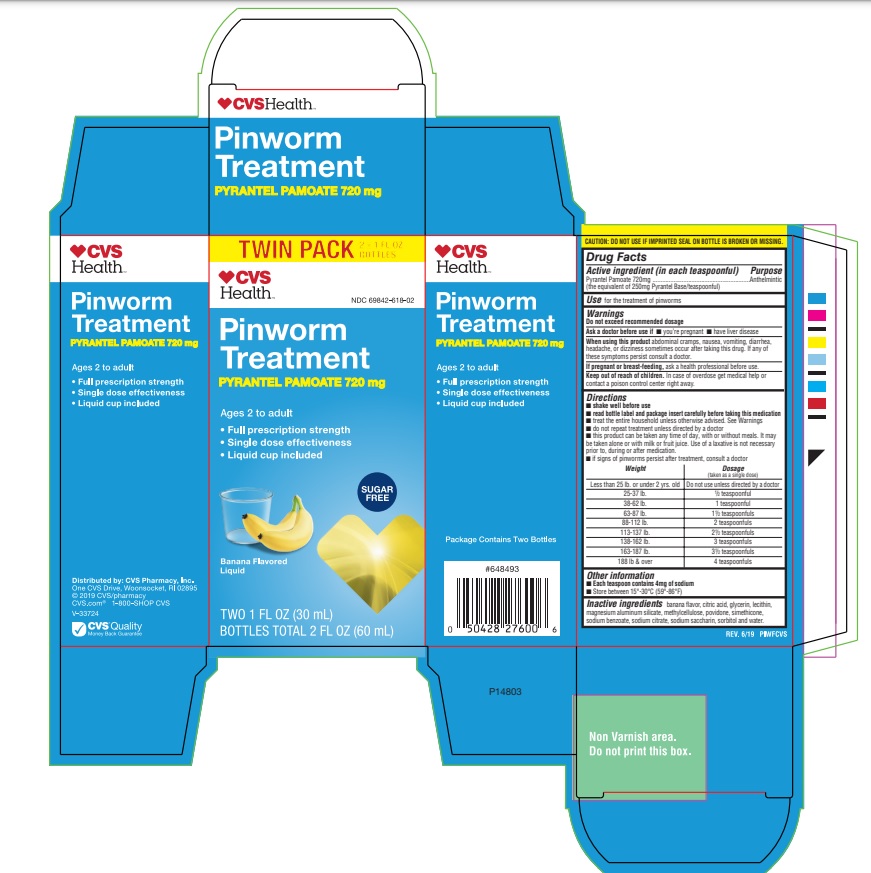
Label: CVS PINWORM TREATMENT- pyrantel pamoate suspension
- NDC Code(s): 69842-618-02
- Packager: CVS PHARMACY INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- shake well before use
- read bottle label and package insert carefully before taking this medication
- treat the entire household unless otherwise advised
- do not repeat treatment unless directed by a doctor
- this product can be taken any time of day, with or without meals. It may be taken alone or with milk or fruit juice. Use of a laxative is not necessary prior to, during or after medication
- it signs of pinworms persist after treatment, consult a doctor
Weight Dosage (taken as a single dose) Less than 25 lb. or under 2 yrs.old Do not use unless directed by a doctor 25-37 lb. 1/2 teaspoonful 38-62 lb. 1 teaspoonful 63-87 lb. 1 1/2 teaspoonfuls 88-112 lb. 2 teaspoonfuls 113-137 lb. 2 1/2 teaspoonfuls 138-162 lb. 3 teaspoonfuls 163-187 lb. 3 1/2 teaspoonfuls 188 lb. and over 4 teaspoonfuls - INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS PINWORM TREATMENT
pyrantel pamoate suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-618 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 144 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) METHYLCELLULOSE (100 CPS) (UNII: 4GFU244C4J) POVIDONE (UNII: FZ989GH94E) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor BANANA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-618-02 2 in 1 CARTON 12/15/2016 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part357B 12/15/2016 Labeler - CVS PHARMACY INC (062312574) Registrant - Reese Pharmaceutical Co (004172052) Establishment Name Address ID/FEI Business Operations LGM Pharma Solutions, LLC. 117549198 manufacture(69842-618) , label(69842-618)