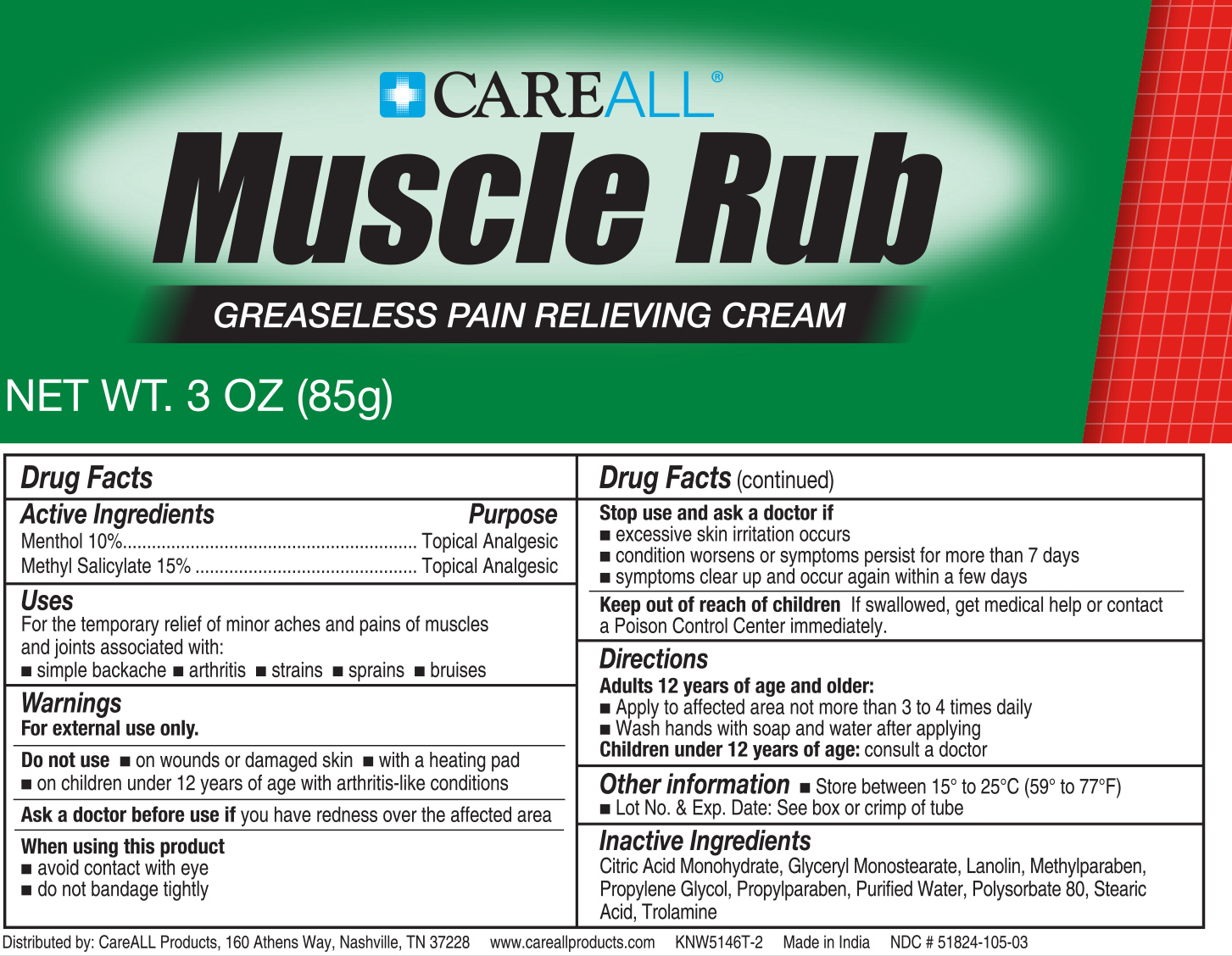
Label: CAREALL MUSCLE RUB ULTRA STRENGTH- menthol, methyl salicylate cream
- NDC Code(s): 51824-105-03
- Packager: New World Imports, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
For external use only
Do not use:
On wounds or damaged skin
With a heating pad
On a child under 12 years of age with arthritis-like conditions
Ask a doctor before use if
you haveredness over the affected area
When using this product
Avoid contact with eyes
Do not bandage tightly
Stop use and ask a doctor if
Condition worsens or symptoms persist for more than 7 days
Symptoms clear up and occur again within a few days
Excessive skin irritation occurs
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CAREALL MUSCLE RUB ULTRA STRENGTH
menthol, methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51824-105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 100 mg in 1 g Methyl Salicylate (UNII: LAV5U5022Y) (Salicylic Acid - UNII:O414PZ4LPZ) Methyl Salicylate 150 mg in 1 g Inactive Ingredients Ingredient Name Strength Citric Acid Monohydrate (UNII: 2968PHW8QP) Glyceryl Monostearate (UNII: 230OU9XXE4) Methylparaben (UNII: A2I8C7HI9T) Propylene Glycol (UNII: 6DC9Q167V3) Propylparaben (UNII: Z8IX2SC1OH) Water (UNII: 059QF0KO0R) Stearic Acid (UNII: 4ELV7Z65AP) Trolamine (UNII: 9O3K93S3TK) LANOLIN (UNII: 7EV65EAW6H) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color white (White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51824-105-03 85 g in 1 TUBE; Type 0: Not a Combination Product 12/12/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/12/2023 Labeler - New World Imports, Inc (075372276)