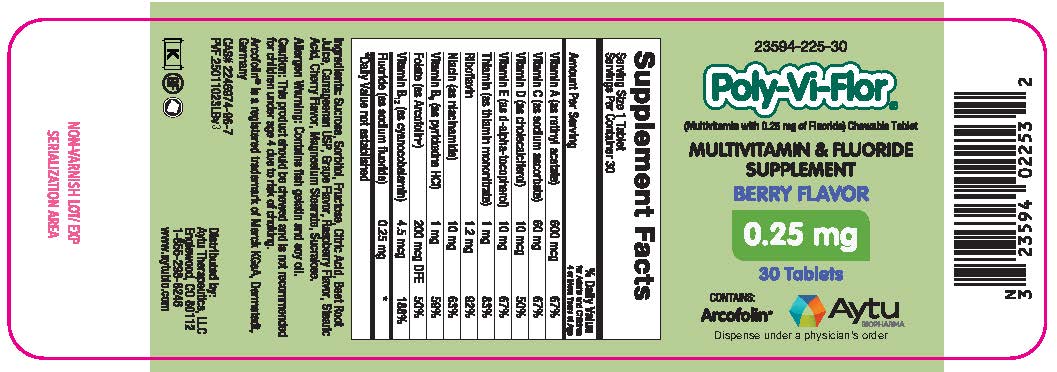
Label: POLY-VI-FLOR- multivitamin and fluoride supplement tablet, chewable
- NHRIC Code(s): 23594-225-30
- Packager: Aytu Therapeutics LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated March 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Poly-Vi-Flor
-
Health Claim
Multivitamin and Fluoride Supplement
Supplement Facts
Serving Size 1 Tablet
Servings Per Container 30Amount Per Serving % Daily Value
for Adults and Children
4 or More Years of AgeVitamin A (as retinyl acetate) 600 mcg 67% Vitamin C (as sodium ascorbate)
60 mg 67% Vitamin D (as cholecalciferol)
10 mcg 50% Vitamin E (as d-alpha-tocopherol) 10 mg 67% Thiamin (as thiamin mononitrate) 1 mg 83% Riboflavin 1.2 mg 92% Niacin (as niacinamide) 10 mg 63% Vitamin B6 (as pyridoxine HCl) 1 mg 59% Folate (as Arcofolin®) 200 mcg DFE 50% Vitamin B12 (as cyanocobalamin) 4.5 mcg 188% Fluoride (as sodium fluoride) 0.25 mg * *Daily Value not established Ingredients: Sucrose, Sorbitol, Fructose, Citric Acid, Beet Root Juice, Carrageenan USP, Grape Flavor, Raspberry Flavor, Stearic Acid, Cherry Flavor, Magnesium Stearate, Sucralose.
- Warnings
- Dosage and Administration
- Safe Handling Warning
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
POLY-VI-FLOR
multivitamin and fluoride supplement tablet, chewableProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:23594-225 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 600 ug SODIUM ASCORBATE (UNII: S033EH8359) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 60 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 10 ug .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 10 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (Thiamine ION - UNII:4ABT0J945J) THIAMINE 1 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.2 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 10 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 4.5 ug SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.25 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) SORBITOL (UNII: 506T60A25R) FRUCTOSE (UNII: 6YSS42VSEV) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CARRAGEENAN (UNII: 5C69YCD2YJ) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:23594-225-30 1 in 1 PACKAGE 1 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 03/14/2023 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color flavor scoring 1 shape size (solid drugs) 10 mm Labeler - Aytu Therapeutics LLC (117244733)