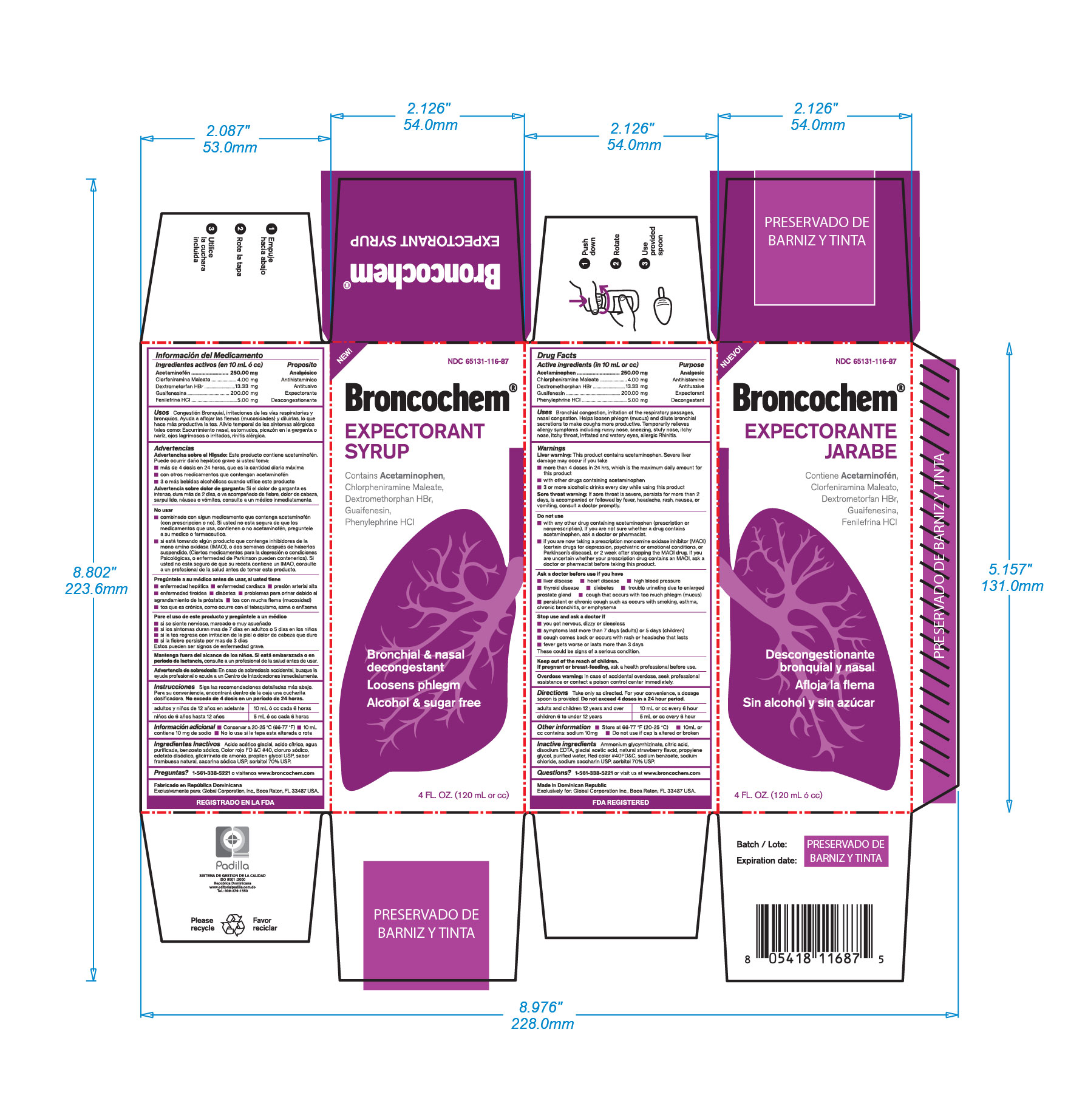
Label: BRONCOCHEM EXPECTORANT II- acetaminophen-chlorpheniramine maleate-dextromethorphan hbr-guaifenesin-phenylephrine hcl syrup
- NDC Code(s): 65131-116-87
- Packager: LABORATORIO MAGNACHEM INTERNATIONAL SRL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 19, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Warnings
Unless directed by a physician do not take this product if persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, emphysema, or if cough is accompanied by excessive phlem (mucus). Likewise if you have heart disease, high blood pressure, thyroid disease, diabetes, or difficulty in urination due to enlargement of the prostate gland, if nervousness, dizziness, or sleeplesness occur, discontinue use or consult a doctor, if symptoms do not improve within 7 days or are accompainied by fever, consult a doctor. A persistent cough may be a sing of a serious condition. Stop use and ask a doctor if symptoms persist or last long that 5 days (children) or 7 days (adults), tend to return, arash, or persistent headache. As with any drug, if you are pregnant or nursing a baby; seek the advice of a healh professional before using this product. Unless directed by a doctor, do not take this product if you are presently taking another product containing Pseudoephedrine HCl, or if you are taking sedatives or trankilizers; it may increase the drowsiness effect. Avoid alcoholic beverages while taking this product. Use caution when driving a motor vehicle or operating machinery.
- Active Ingredients
- Purpose
- Keep Out Of the Reach Of children
-
Indications & Use
Bronchial congestion
Irritation of the respiratory passages
Nasal congestion
Helps loosen phlegm (mucus) and dilute bronchial secretions to make coughs more productive
Antitussive
Temporarilly relieves allergy symptoms including runny nose, sneezing, stuffy nose, itchy nose, itchy throat, irritated and watery eyes, allergic rhinitis
- Dosage and Administration
-
Drug Interaction Precautions
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI). (certain drugs for depression, psychiatric or emotional conditions, or Parkinson`s disease), or 2 weeks after stopping the MAOI drug. If you are uncertain whether your presciption drug contains an MAOI, consult a health professional before taking this product
- Iactive Ingredient
- Package Label Principal Display Panel
- Iactive Ingredient
-
INGREDIENTS AND APPEARANCE
BRONCOCHEM EXPECTORANT II
acetaminophen-chlorpheniramine maleate-dextromethorphan hbr-guaifenesin-phenylephrine hcl syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65131-116 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg in 10 mL CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg in 10 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 13.33 mg in 10 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 10 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE 5 mg in 10 mL Inactive Ingredients Ingredient Name Strength SACCHARIN SODIUM (UNII: SB8ZUX40TY) 40 mg in 10 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 20 mg in 10 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 12 mg in 10 mL AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) 6 mg in 10 mL DISODIUM HEDTA (UNII: KME849MC7A) 5 mg in 10 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 2 mg in 10 mL SORBITOL (UNII: 506T60A25R) 2.3 mL in 10 mL PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 2 mL in 10 mL ACETIC ACID (UNII: Q40Q9N063P) 0.016 mL in 10 mL RASPBERRY (UNII: 4N14V5R27W) 0.006 mL in 10 mL FD&C RED NO. 40 (UNII: WZB9127XOA) 0.29 mg in 10 mL WATER (UNII: 059QF0KO0R) 10 mL in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65131-116-87 1 in 1 BOX 12/30/2016 1 120 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 12/30/2016 Labeler - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) Registrant - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) Establishment Name Address ID/FEI Business Operations LABORATORIO MAGNACHEM INTERNATIONAL SRL 871446100 manufacture(65131-116)