Label: metvixia- methyl aminolevulinate hydrochloride cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 63069-401-01 - Packager: Penn Pharmaceutical Services Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 26, 2007
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
-
DESCRIPTION
Metvixia Cream is an oil in water emulsion. Metvixia Cream contains methyl aminolevulinate hydrochloride equivalent to168 mg/g of methyl aminolevulinate.
Methyl aminolevulinate hydrochloride is a white to slightly yellow powder that is freely soluble in water and methanol, soluble in ethanol and practically insoluble in most organic solvents.
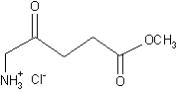
The chemical formula for methyl aminolevulinate HCl is C6H11NO3·HCl (MW=181.62) and it has the following structural formula:
Metvixia Cream contains glyceryl monostearate, cetostearyl alcohol, polyoxyl stearate, cholesterol and oleyl alcohol as emulsifying agents. It also contains glycerin, white petrolatum, isopropyl myristate, refined peanut oil, refined almond oil as emollients, edetate disodium as a chelating agent and methylparaben and propylparaben as preservatives.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Photosensitization following application of Metvixia Cream occurs through the metabolic conversion of methyl aminolevulinate (prodrug) to photoactive porphyrins (PAP), which accumulates in the skin lesions to which Metvixia Cream has been applied. When exposed to light of appropriate wavelength and energy, the accumulated photoactive porphyrins produce a photodynamic reaction, resulting in a cytotoxic process dependent upon the simultaneous presence of oxygen. The absorption of light results in an excited state of porphyrin molecules, and subsequent spin transfer from photoactive porphyrins to molecular oxygen generates singlet oxygen, which can further react to form superoxide and hydroxyl radicals. Photosensitization of actinic (solar) keratosis lesions using Metvixia Cream, plus illumination with a CureLight BroadBand Model CureLight 01 (a red light of 570 to 670 nm wavelength) at 75 J/cm2, is the basis for Metvixia photodynamic therapy (PDT).
Pharmacokinetics
The time-course of PAPs after application of Metvixia Cream has been monitored by means of fluorescence. After application of Metvixia Cream to actinic keratosis lesions in 8 patients, fluorescence was measured at several time points over 28 hours. Three hours after the application of Metvixia Cream the fluorescence in the treated lesions was significantly greater than that seen in both treated and untreated normal skin, and after application of vehicle cream (not containing methyl aminolevulinate) to normal skin. After application of Metvixia Cream for 28 hours and subsequent illumination with red light of 570 to 670 nm wavelength at a total light dose of 75 J/cm2, complete photobleaching (photodegradation) of Protoporphyrin IX occurred with levels of Protoporphyrin IX returning to pre-treatment values within 1 hour after illumination. However, the fate of other photoactive porphyrins are unknown.
The clinical dose of methyl aminolevulinate cream and duration of application were derived from a study in which three different strengths of the cream (16, 80 and 160 mg/g methyl aminolevulinate, as hydrochloride), each applied for 3 hours or 18 hours, were tested in 16 patients.
CLINICAL STUDIES
Metvixia Cream plus illumination with the CureLight BroadBand Model CureLight 01 (a red light of 570 to 670 nm wavelength) at 75 J/cm2 has been studied in 130 patients with non-hyperkeratotic actinic keratoses in two clinical trials. These trials were not identical; however, both were randomized, multicenter, and double-blinded with patients randomized to Metvixia-PDT and Vehicle-PDT study arms that required two treatment sessions (7 days apart). One study was conducted in the U.S. and patients were randomized 1:1 Metvixia to Vehicle and one study was conducted in Australia with patients randomized 4:1 Metvixia to Vehicle. In both studies treatment consisted of a multi-step process that was repeated after 7 days consisting of 1) Lesion preparation (debridement with sharp curette) to roughen the surface of the lesion. 2) Metvixia or Vehicle Cream application to lesions with occlusion with an adhesive, non-absorbent dressing, 3) Waiting at least 2.5 hours, but no more than 4 hours to allow for conversion of the methyl aminolevulinate, 4) Removal of cream with gauze and saline, 5) Red light Dosimetry and Illumination with the CureLight BroadBand Model CureLight 01 (a red light of 570 to 670 nm wavelength).
Study patients had previously untreated facial and scalp actinic keratoses (AKs) that were slightly palpable (better felt than seen). Hyperkeratotic actinic keratoses were excluded. In the U.S. study 100% of patients had 4 to 10 lesions at baseline. However, in the Australian study, 63% (70/111) of patients had less than 4 lesions at baseline, 31% (34/111) of the enrolled patients had 4 to 10 lesions, and 6% (7/111) had more than 10 lesions at baseline (a maximum of 6 treatment fields were allowed in this study).
A “Cleared” AK lesion was defined as being not visible and not palpable as assessed 3 months after the second treatment session. Patients with all treated lesions cleared at 3 months were defined as Complete Responders.
The percentage of patients in whom 75% or more of the treated lesions were clear and the percent of patients in whom 100% of the treated lesions were clear 3 months after the second Metvixia-PDT treatment session are shown in Table 1 for each of the two studies.
Table 1. Patient Responses at 3-Month Post-Second Treatment Session Australian Study U. S. Study Patient Response Metvixia-PDT Vehicle PDT Metvixia -PDT Vehicle PDT Patients with at least 75% of AK Lesions Cleared 76/88 (86%) 4/23 (17%) 35/42 (83%) 12/38 (32%) Complete Responders (All Lesions clinically cleared at 3 months) 71/88 (81%) 3/23 (13%) 33/42 (79%) 8/38 (21%) Patients with 4 or more lesions had lower success rates than those with less than 4 lesions when treated with PDT using Metvixia Cream (see Table 2).
Table 2. Complete Responders at 3-Month Post-Treatment for Different Baseline AK Lesion Counts Australian Study
U.S. Study
Baseline Lesion Count Metvixia-PDT Vehicle PDT Metvixia-PDT Vehicle PDT Below 4 49/55 (89%) 3/15 (20%) N/A N/A 4 – 10 18/27 (67%) 0/7 33/42 (79%) 8/38 (21%) More than 10 4/6 (67%) 0/1 N/A N/A Total 71/88 (81%) 3/23 (13%) 33/42 (79%) 8/38 (21%) Lesions that were slightly palpable (Grade 1) had a better success rate than lesions that were visible and palpable (Grade 2) – See Table 3.
Table 3. Lesion Complete Response at 3-Month Post-Treatment for Different Lesion Grades Australian Study
U.S. Study
Lesion Grade Metvixia-PDT Vehicle PDT Metvixia-PDT Vehicle PDT Lesion Grade 1 (slightly palpable AK: better felt than seen) 198/209 (95%) 12/35 (34%) 172/196 (88%) 72/162 (44%) Lesion Grade 2 (visible and palpable AK: easily seen and felt) 119/151 (79%) 9/39 (23%) 49/64 (77%) 20/80 (25%) Total 317/360 (88%) 21/74 (28%) 221/260 (85%) 92/242 (38%) Response rate by lesion location is presented in Table 4.
Table 4. Lesion Complete Response at 3-Month Post-Treatment for Different Lesion Locations Australian Study
U.S. Study
Lesion Location Metvixia-PDT Vehicle PDT Metvixia-PDT Vehicle PDT Face 251/273 (92%) 15/62 (24%) 195/226 (86%) 73/204 (36%) Scalp 66/87 (76%) 6/12 (50%) 26/34 (76%) 19/38 (50%) Total 317/360 (88%) 21/74 (28%) 221/260 (85%) 92/242 (38%) The overall treatment effect as evidenced by the high vehicle response rate may include the contribution of lesion preparation, i.e. curettage. Adequate lesion preparation is an important component for AK therapy with Metvixia Cream.
Information regarding further treatments for residual or new AK lesions performed after 3 months is not available.
-
INDICATIONS AND USAGE
Metvixia Cream in combination with 570 to 670 nm wavelength red light illumination using the CureLight BroadBand Model CureLight 01 lamp is indicated for treatment of non-hyperkeratotic actinic keratoses of the face and scalp in immunocompetent patients when used in conjunction with lesion preparation (debridement using a sharp dermal curette) in the physician’s office when other therapies are unacceptable or considered medically less appropriate.
-
CONTRAINDICATIONS
Metvixia Cream is contraindicated in patients with cutaneous photosensitivity, or known allergies to porphyrins, and in patients with known sensitivities to any of the components of Metvixia Cream, which includes peanut and almond oil (See WARNINGS regarding sensitivity to Metvixia Cream).
This product contains refined peanut oil (See PRECAUTIONS).
-
WARNINGS
Metvixia Cream is intended for topical use in the physician’s office by trained physicians only. Do not apply to the eyes or to mucous membranes.
Metvixia Cream has demonstrated a high rate of contact sensitization (allergenicity) (See ADVERSE REACTIONS). Care should be taken by the physician applying Metvixia Cream to avoid inadvertent skin contact. Nitrile gloves should be worn when applying and removing the cream. Vinyl and latex gloves do not provide adequate protection when using this product.
Metvixia Cream when used with CureLight BroadBand Model CureLight 01 lamp must be used with appropriate protective sleeves obtained from the product manufacturer to decrease the risk of blood-borne transmitted diseases (hepatitis, HIV, etc.). Change the disposable covers for the device (probe and horseshoe positioning device) between patients. Universal Precautions should be used with this treatment.
-
PRECAUTIONS
The safety and efficacy have not been established for the treatment of cutaneous malignancies and for skin lesions other than non-hyperkeratotic face and scalp actinic keratoses using PDT with Metvixia Cream. Thick (hyperkeratotic) actinic keratoses should not be treated with Metvixia Cream. The safety and efficacy of Metvixia Cream has not been established in patients with immunosuppression, porphyria or pigmented actinic keratoses.
General
Metvixia Cream Application
During the time period between the application of Metvixia (methyl aminolevulinate) Cream and exposure to red light illumination, the treatment site will become photosensitive. After Metvixia Cream application, patients should avoid exposure of the photosensitive treatment sites to sunlight or bright indoor light (e.g., examination lamps, operating room lamps, tanning beds, or lights at close proximity) during the period prior to red light treatment. Exposure to light may result in a stinging and/or burning sensation and may cause erythema and/or edema of the lesions. Before exposure to sunlight, patients should, therefore, protect treated lesions from the sun by wearing a wide-brimmed hat or similar head covering of light-opaque material. Sunscreens will not protect against photosensitivity reactions caused by visible light. The treated site should be protected from extreme cold with adequate clothing or remaining indoors between application of Metvixia and PDT light treatment.
After illumination of Metvixia Cream, the area treated should be kept covered and away from light for at least 48 hours.
Because of the potential for skin to become photosensitized, the Metvixia Cream should be used by a trained physician to apply drug only to non-hyperkeratotic actinic keratoses and perilesional skin within 5 mm of the lesion. Redness, swelling, burning, and stinging are expected as a result of therapy; however, if these symptoms increase in severity and persist longer than 3 weeks, the patient should contact their doctor.
Metvixia Cream has not been studied for more than two treatment sessions.
Information regarding further treatments for residual or new AK lesions performed after 3 months is not available.
.
Photosensitivity and Device Precautions.
The patient, operator and other persons present should wear protective goggles that sufficiently screen out light with wavelengths from 570 to 670 nm during red light treatment.
If for any reason the patient cannot have the red light treatment after application of Metvixia Cream, the cream should be rinsed off, and the patient should protect the treated area from sunlight, prolonged or intense light for two days. Prolonged exposure for greater than 4 hours to Metvixia Cream should be avoided.
Coagulation defects
Metvixia Cream has not been tested on patients with inherited or acquired coagulation defects.
Hypersensitivity
Metvixia Cream is formulated with refined peanut and almond oil.
Metvixia (methyl aminolevulinate) Cream has not been tested in patients who are allergic to peanuts. Metvixia (methyl aminolevulinate) Cream has demonstrated a high rate of contact sensitization (allergenicity).
Information for Patients
The physician should provide and discuss the attached Patient Package Insert with each patient.
Drug Interactions
There have been no studies of the interaction of Metvixia Cream with any other drugs, including local anesthetics. It is possible that concomitant use of other known photosensitizing agents might increase the photosensitivity reaction of actinic keratoses treated with Metvixia Cream.
Carcinogenesis, Mutagenesis, Impairment to Fertility
Long-term studies to evaluate the carcinogenic potential of Metvixia Cream have not been performed.
Methyl aminolevulinate was negative for genetic toxicity in the Ames assay, and the chromosomal aberration assay in Chinese hamster ovary cells, tested with and without metabolic activation and in the presence and absence of light. Methyl aminolevulinate was also negative in the in vivo micronucleus assay in the rat. In contrast, at least one report in the literature has noted genotoxic effects in cultured rat hepatocytes after aminolevulinate (ALA) exposure with PpIX formation. Other studies have documented oxidative DNA damage in vivo and in vitro as a result of ALA exposure.
No animal fertility studies have been conducted.
Pregnancy
Teratogenic effects
Pregnancy Category C: Animal reproduction studies have not been conducted with Metvixia Cream. It is also not known whether Metvixia Cream can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity.
Metvixia Cream should be given to a pregnant woman only if clearly needed.
Nursing Mothers
The amount of methyl aminolevulinate secreted into human breast milk following topical administration of Metvixia Cream is not known. Because many drugs are secreted in human milk, caution should be exercised when Metvixia Cream is administered to a nursing mother. If Metvixia Cream is used in a nursing mother, a decision should be made whether or not to stop nursing.
-
ADVERSE REACTIONS
Dermal Safety Studies
Provocative studies to evaluate irritancy and sensitization have demonstrated that Metvixia Cream is an irritant and sensitizer. A provocative cumulative irritancy and sensitization (allergenicity) study of Metvixia Cream with a cross-sensitization challenge with ALA was performed in 156 subjects. Only 98 of the 156 subjects tested entered the challenge phase. Fifty-two percent of the subjects (30/58), who agreed to challenge with Metvixia Cream, were positive (sensitized). Forty subjects refused challenge with Metvixia Cream and 60 withdrew. At least 58 of the 60 subjects who withdrew from the study discontinued due to irritation/sensitization.
Ninety-eight subjects agreed to challenge with ALA. Two percent of the ALA challenged subjects (2/98) were scored as equivocal reactions and 2% in the paraffin vehicle group were scored as positive.
Adverse Events
In vehicle-controlled phase 3 studies of actinic keratosis, 88% of patients treated with Metvixia Cream reported one or more adverse events.
Burning was the most frequent complaint, reported by 50% of patients (ranging from mild, to severe) and 9% of those patients reported severe burning sensation. Pain in the skin was reported by 21% of patients and 7% had severe pain. Local erythema lasting up to two weeks and edema up to one week after treatment were reported by 31% and 6% of patients.
Symptoms and signs of local phototoxicity were observed in 88% of patients treated with Metvixia Cream in all clinical studies of Metvixia -PDT for actinic keratoses.
Percentage of patients with local adverse reactions based on occurrence per patient in vehicle controlled phase 3 studies. Events Metvixia-PDT
(n=130)Vehicle PDT*
(n=61)n (%) of patients with AEs n (%) of patients with AEs Burning sensation skin 65 (50.0%) 9 (14.8%) Erythema 60 (46.2%) 12 (19.7%) Skin pain 27 (20.8%) 6 (9.8%) Stinging skin 25 (19.2%) 2 (3.3%) Crusting 20 (15.4%) 6 (9.8%) Edema skin 20 (15.4%) 1 (1.6%) Skin peeling 14 (10.8%) 2 (3.3%) Blisters 14 (10.8%) 2 (3.3%) Bleeding skin 11 (8.5%) 2 (3.3%) Pruritus/Itching 17 (13.1%) 2 (3.3%) Skin ulceration 7 (5.4%) 0 (0%) Skin infection 3 (2.3%) 1 (1.6%) Skin hyper-pigmentation 1 (0.8%) 0 (0%) The majority of patients in all the clinical trials had local pain or discomfort upon illumination. There were 4 (1.0%) withdrawals/discontinuations among 383 patients treated with Metvixia Cream in all the clinical trials of actinic keratosis, all of which were due to the adverse event of local pain on illumination.
There have been reported instances of patients treated with Metvixia Cream (2 out of 130) who have developed squamous cell and basal cell carcinoma at the site of treatment. The relationship to treatment with Metvixia Cream is unknown.
Serious erythema and facial edema have been described in European post-marketing reports.
-
OVERDOSAGE
Metvixia Cream Overdose
Metvixia Cream overdose has not been reported. If the patient for any reason cannot have the red light treatment during the prescribed period after application (the 3 hour timespan), the cream should be rinsed off, and the patient should protect the exposed area from sunlight, prolonged or intense light for two days.
-
DOSAGE AND ADMINISTRATION
Photodynamic therapy for non-hyperkeratotic actinic keratoses with Metvixia Cream is a multi-stage process as described below: Two treatment sessions 7 days apart should be conducted. Not more than one gram (half a tube) of Metvixia Cream should be applied per treatment session.
One Metvixia -PDT session consists of:
1) Lesion debriding –
Before applying Metvixia Cream, the surface of the lesions should be prepared with a small dermal curette to remove scales and crusts and roughen the surface of the lesion. This is to facilitate access of the cream and light to all parts of the lesion.
Figure 1 A Lesion debridingOnly nitrile gloves should be worn during this and subsequent steps and Universal Precautions should be taken. Vinyl and latex gloves do not provide adequate protection when using this product.

Figure 1B Lesion debriding2) Application of Metvixia Cream –
Using a spatula, apply a layer of Metvixia Cream about 1 mm thick to the lesion and the surrounding 5 mm of normal skin. Do not apply more than one gram of Metvixia Cream for each patient per treatment session.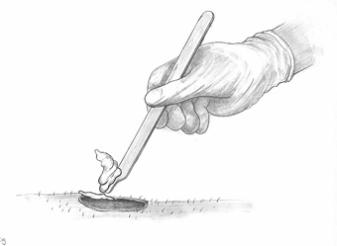
Figure 2: Cream applicationThe area to which the cream has been applied should then be covered with an occlusive, non-absorbent dressing for 3 hours. Multiple lesions may be treated during the same treatment session. Each treatment field is limited to a diameter of 55 mm. Only nitrile gloves should be worn by the qualified healthcare provider in order to avoid skin contact with the cream. This product is not intended for application by patients or unqualified medical personnel.
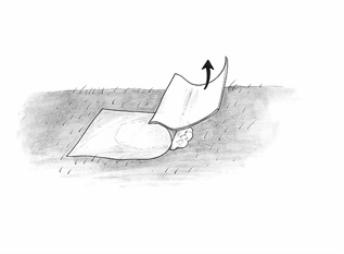
Figure 3: Occlusive dressing application3) Wait for 3 hours - (at least 2.5 hours, but no more than 4 hours).
After Cream application, patients should avoid exposure of the photosensitive treatment sites to sunlight or bright indoor light (e.g., examination lamps, operating room lamps, tanning beds, or lights at close proximity) during the period prior to red light treatment. Exposure to light may result in a stinging and/or burning sensation and may cause erythema and/or edema of the lesions. Patients should protect treated areas from the sun by wearing a wide-brimmed hat or similar head covering of light-opaque material. Sunscreens will not protect against photosensitivity reactions caused by visible light. It has not been determined if perspiration can spread the Metvixia Cream outside the treatment site to the eyes or surrounding skin. The treated site should be protected from extreme cold with adequate clothing or remaining indoors between application of Metvixia Cream and PDT light treatment.4) Removal of Dressing and Rinse Off Excess Cream - Following removal of the occlusive dressing, clean the area with saline and gauze. Nitrile gloves should be worn at this step by the trained physician.
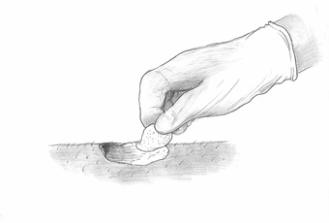
Figure 4: Cream removal5) Illumination of Metvixia Treated Lesion - It is important to ensure that the correct light dose is administered. The light intensity at the lesion surface should not be higher than 200 mW/cm2. Patient and operator should adhere to safety instructions and Universal Precautions provided with the lamp. The patient and operator should wear protective goggles during illumination. Patients should be advised that transient stinging and/or burning at the target lesion sites may occur during the period of light exposure.
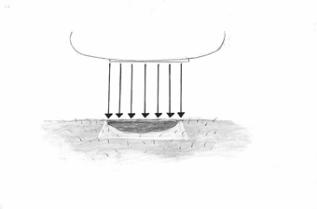
Figure 5: IlluminationThe CureLight BroadBand Model CureLight 01 lamp is approved for the use in Metvixia -PDT. The lamp should be carefully calibrated so that dosing is accurate and immediately thereafter the lesion should be exposed to red light with a continuous spectrum of 570 to 670 nm and a total light dose of 75 J/cm2. To avoid direct contact between lamp parts and patient skin, always use disposable protective plastic sleeves on the positioning device and on the light measuring probe. Following each patient treatment, the disposable protective plastic sleeves should be removed from the positioning device and from the light measuring probe and discarded.
If red light treatment is interrupted or stopped for any reason, it may be restarted. If the patient for any reason cannot have the red light treatment during the prescribed period after application (the 3 hour timespan), the cream should be rinsed off and the patient should protect the exposed area from sunlight, prolonged or intense light for two days. Metvixia Cream is not intended for use with any device other than the approved lamp: CureLight BroadBand Model CureLight 01.
Use of Metvixia Cream without subsequent red light illumination is not recommended.
No more than 1 gram (half a tube) of product should be used for each of the two weekly treatment sessions. Multiple lesions may be treated during the same treatment session using a total of 1 gram of Metvixia Cream. Lesion response should be assessed 3 months after the last treatment session.
This product is not intended for application by patients or unqualified medical personnel, therefore, this product is only dispensed to physicians.
-
HOW SUPPLIED
Metvixia Cream, 16.8%, is available as the following:
NDC 63069-401-01, 2 gram aluminum tube, box of 1
Product Package
Keep out of reach of children
For topical use only by physicians in the physician’s office.
Rx Only
-
SUPPLEMENTAL PATIENT MATERIAL
PATIENT INFORMATION
Metvixia™ Cream 16.8% (phonetic)
Generic name: methyl aminolevulinate hydrochlorideRead this Patient Information before you get treated with Metvixia Cream and each time you get a treatment. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or treatment. Ask your healthcare provider about anything you do not understand about Metvixia Cream.
What is the most important thing I need to know about Metvixia Cream?
- Metvixia Cream with light treatment (Photodynamic therapy or PDT) is only done in medical offices by trained doctors.
- Metvixia Cream is not applied by patients. Metvixia Cream should not be applied by doctors who have not been trained in its use.
What is Metvixia Cream?
Metvixia Cream is a prescription cream used with PDT (light treatment) to treat skin growths on the face and scalp called actinic keratosis (AK). Metvixia Cream is only used for AK skin growths that are thin and not dark colored. AK skin growths are not cancer. AK skin growths are caused partly by too much sun exposure. Metvixia Cream and PDT work together to treat AK skin growths.
Metvixia Cream has not been studied in children for any condition and should not be used in children.
Who should not use Metvixia Cream?
Do not use Metvixia Cream if:
- -
- your skin over reacts to sun or light (photosensitivity)
- -
- you are allergic to porphyrins or to any of the ingredients in Metvixia Cream. The active ingredient is methyl aminolevulinate hydrochloride. Metvixia Cream also contains peanut and almond oil. See the end of this leaflet for a complete list of ingredients in Metvixia Cream.
Before treatment with Metvixia Cream, tell your doctor:
-
about your medical conditions, including if you
- are pregnant or planning to become pregnant. It is not known if Metvixia Cream can harm your unborn baby.
- are breastfeeding. It is not known if Metvixia Cream passes into your milk and if it can harm your baby. You should decide whether or not to stop breastfeeding while getting treatment with Metvixia Cream. Talk to your doctor for help with this choice.
- are allergic to nuts or peanuts
- have or had skin cancer or other skin growths on your body
- have bleeding problems.
- about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. It is not known if Metvixia Cream and other medicines can affect each other.
How should I use Metvixia Cream?
- Metvixia Cream and PDT treatment is only done by trained doctors.
- You will receive 2 treatments with Metvixia Cream and PDT 7 days (1 week) apart. Your doctor will check you three months after treatment to see if the treatment worked for you. (See the end of this leaflet for the section “Treatment with Metvixia Cream and PDT.”)
- Metvixia Cream is for skin use only. Do not get Metvixia Cream in your eyes, mouth, or nose. Tell your doctor right away if this happens.
What should I avoid while using Metvixia Cream?
During the 3 hours that Metvixia Cream is on your skin:
- Avoid exposure to sunlight or bright indoor light during the 3 hours that Metvixia Cream is on your skin. Wear a protective hat and clothing if you need to be outside in the sun.
- Avoid exposure to cold temperatures during the 3 hours that Metvixia Cream is on your skin. Wear warm clothing and keep your treated skin site covered if you are in cold temperatures.
If for some unavoidable reason you are not treated with the lamp you should
- Carefully rinse off the Cream.
- Avoid exposure to sunlight, prolonged or intense light for two days after treatment.
What are the possible side effects of Metvixia Cream with PDT treatment?
Common side effects of Metvixia Cream with PDT treatment include the following skin reactions at the treated site:
- burning feeling
- redness
- pain
- stinging
- swelling
- crusting, peeling, blisters, bleeding, itching, ulcers
- infection
Tell your doctor if you get any of these side effects. Your healthcare provider should be able to treat these reactions according to standard treatments for such skin reactions. These reactions usually go away within 10 days of treatment. Redness may last for up to 1 month. If any of your skin reactions get worse and last longer than 3 weeks, call your doctor.
These are not all the side effects of Metvixia Cream with PDT. Ask your doctor or pharmacist for more information.
General information about Metvixia Cream
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets.
This leaflet summarizes the most important information about Metvixia Cream. If you would like more information, talk with your doctor. You can ask your doctor for information about Metvixia Cream that is written for health professionals.
Toll-free number and/or website will be provided when available for the US market.
What are the ingredients in Metvixia Cream?
Active Ingredient: methyl aminolevulinate hydrochloride
Other Ingredients: Glyceryl monostearate, cetostearyl alcohol, poloxyl stearate, cholesterol, oleyl alcohol glycerin, white petrolatum, isopropyl myristate, refined peanut oil, refined almond oil, edetate disodium, methylparaben and propylparaben. The color of the product is cream to pale yellow.
Treatment with Metvixia Cream and PDT

Figure 1: Lesion debridingYour doctor will prepare your skin by gently scraping (debriding) your skin growths before treating with Metvixia Cream and PDT. A small skin scraper is used to remove scales and crusts and to roughen the surface of any skin growths. This is to help Metvixia Cream and PDT to reach all parts of the skin growths.

Figure 2: Cream applicationMetvixia Cream is applied to the actinic keratosis skin growths and to a small area of the skin around the growths.

Figure 3: Clear bandage applicationThe treated skin areas will be covered with a special clear bandage for about 3 hours.
During these 3-hours you should avoid exposure of treated area to sunlight or bright indoor light. Exposure to light may make your treated skin area sting or burn. Your treated skin area may turn red or swell (photosensitive reactions). Wear a hat and protective clothes if you are exposed to sunlight during this time. Sunscreens will not help protect your treated skin during this time. In cold weather, your treated skin site should be protected from the cold with warm clothes or you should stay indoors for these 3 hours between the cream and light treatment.

Figure 4: Cream removalThe clear bandage will be removed and the area will be rinsed with a saline solution before the PDT (light) treatment.

Figure 5: IlluminationThe skin growth will be treated with PDT. PDT lasts about 10 minutes for each area treated with the lamp. You will wear protective goggles to cover your eyes during this part of the treatment.
More than 1 skin growth may be treated at a time. Your treated skin areas may burn, feel painful, sting, or tingle during light treatment. These symptoms may last for a few hours after the treatment. If you cannot have the light treatment 3 hours after Metvixia Cream is applied, rinse the cream off your skin and you must protect your skin from sunlight and bright indoor light for 2 days.
This product should only be stored in refrigerators in pharmacies and medical offices.
Rx only
Metvixia Cream is a registered trade name of PhotoCure ASA.
Sponsor: PhotoCure ASA, Hoffsveien 48, NO-0377 Oslo, Norway
U.S. Contact: Cato Research, Westpark Corporate Center, 4364 South Alston Avenue, Durham NC 27713
Manufacturer: Penn Pharmaceutical Services Ltd., Tafarnaubach Industrial Estate, Tredegar, Gwent, NP22 3AA, UK. -
INGREDIENTS AND APPEARANCE
METVIXIA
methyl aminolevulinate hydrochloride creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63069-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength methyl aminolevulinate hydrochloride (UNII: 7S73606O1A) (methyl aminolevulinate - UNII:585NM85KYM) 168 mg in 1 g Inactive Ingredients Ingredient Name Strength glyceryl monosterarate () cetostearyl alcohol () polyoxyl stearate () cholesterol (UNII: 97C5T2UQ7J) oleyl alcohol (UNII: 172F2WN8DV) glycerin (UNII: PDC6A3C0OX) white petrolatum (UNII: 4T6H12BN9U) isopropyl myristate (UNII: 0RE8K4LNJS) refined peanut oil () refined almond oil () edetate disodium (UNII: 7FLD91C86K) methylparaben (UNII: A2I8C7HI9T) propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63069-401-01 2 g in 1 TUBE Labeler - Penn Pharmaceutical Services Ltd.
