Label: BACIGUENT- bacitracin ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 69784-880-01, 69784-880-35 - Packager: Woodward Pharma Services LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 26, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STERILE Rx Only
- DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
-
PRECAUTIONS:
Baciguent® ophthalmic ointment should not be used in deep-seated ocular infections or in those that are likely to become systemic. The prolonged use of antibiotic containing preparations may result in overgrowth of nonsusceptible organisms particularly fungi. If new infections develop during treatment appropriate antibiotic or chemotherapy should be instituted.
-
ADVERSE REACTIONS:
Bacitracin has such a low incidence of allergenicity that for all practical purposes side reactions are practically non-existent. However, if such reaction should occur, therapy should be discontinued.
To report SUSPECTED ADVERSE REACTIONS, contact Woodward Pharma Services LLC at 1-844-200-7910 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION:
The ointment should be applied directly into the conjunctival sac 1 to 3 times daily. In blepharitis all scales and crusts should be carefully removed and the ointment then spread uniformly over the lid margins. Patients should be instructed to take appropriate measures to avoid gross contamination of the ointment when applying the ointment directly to the infected eye.
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
-
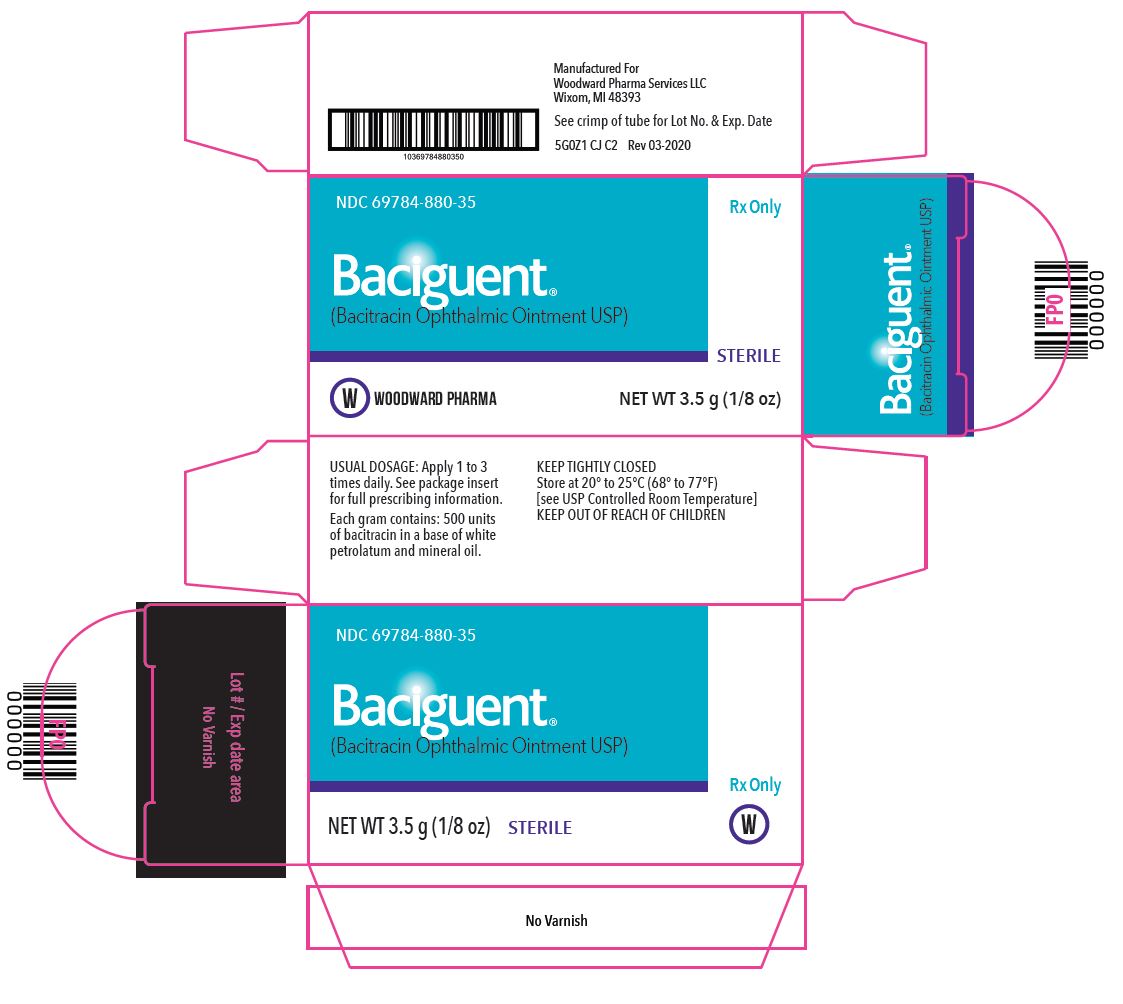
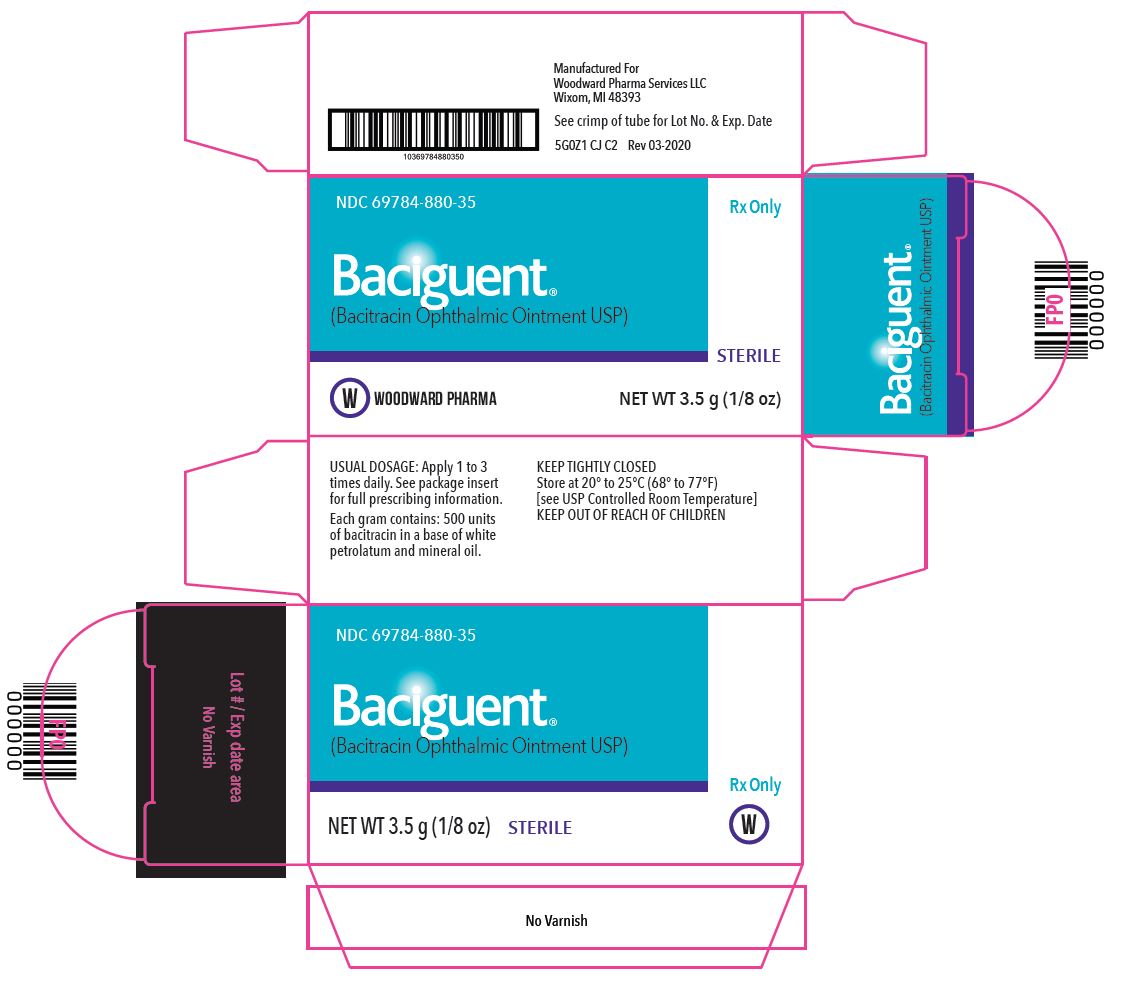
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 1 g Label
Rx Only
NDC 69784-880-35
Baciguent®
(Bacitracin Ophthalmic Ointment, USP)STERILE
NET WT 3.5 g (1/8 oz)
USUAL DOSAGE: Apply 1 to 3 times daily. See package insert for full prescribing information.
Each gram contains: 500 units of bacitracin in a base of white petrolatum and mineral oil.
KEEP TIGHTLY CLOSED
Store at 20° to 25°C (68°-77°F) [see USP Controlled Room Temperature].
KEEP OUT OF REACH OF CHILDREN
-
INGREDIENTS AND APPEARANCE
BACIGUENT
bacitracin ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69784-880 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN (UNII: 58H6RWO52I) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69784-880-01 3 in 1 CARTON 04/15/2020 1 1 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69784-880-35 1 in 1 CARTON 04/15/2020 2 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA061212 04/15/2020 Labeler - Woodward Pharma Services LLC (026749066) Registrant - Woodward Pharma Services LLC (026749066)