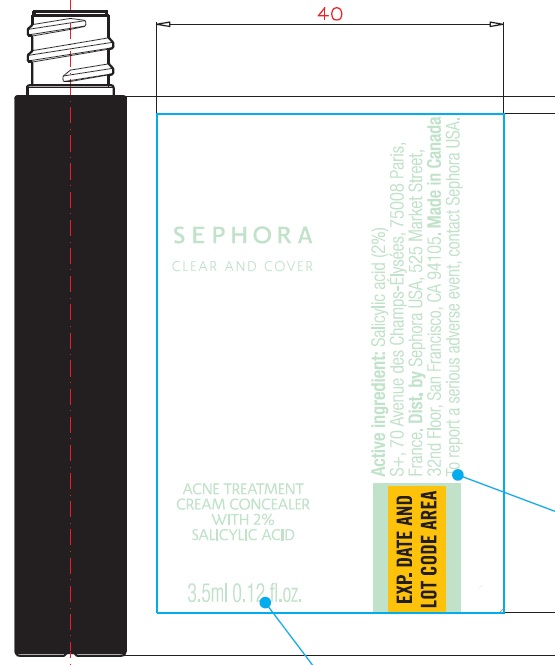
Label: CLEAR AND COVER ACNE TREATMENT CONCEALER CLEAR AND COVER ACNE TREATMENT CONCEALER 13 FUDGE- salicylic acid cream
- NDC Code(s): 31720-965-18
- Packager: S+
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
- Warnings
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- becaused excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three tiems daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
-
Inactive ingredients
Water (Aqua), Cyclopentasiloxane, Butylene Glycol, Dimethicone, Trisiloxane, Kaolin, Polyisobutene, PEG/PPG-18/18 Dimethicone, Boron Nitride, Cyclodextrin, Talc, Cetyl PEG/PPG-10/1 Dimethicone, Disteardimonium Hectorite, Magnesium Sulfate, Methyl Methacrylate Crosspolymer, Trimethylsiloxysilicate, Phenoxyethanol, Bisabolol, Caprylyl Glycol, Glycerin, Triethoxycaprylylsilane, Chamomilla Recutita (Matricaria) Flower Extract, Tocopherol, Tocopheryl Acetate, Pentaerythrityl Tetra-Di-T-Butyl Hydroxyhydrocinnamate.
May Contain (+/-): Zinc Oxide (CI 77947), Iron Oxides (CI 77491/CI 77492/CI 77499). - Package Labeling:
-
INGREDIENTS AND APPEARANCE
CLEAR AND COVER ACNE TREATMENT CONCEALER CLEAR AND COVER ACNE TREATMENT CONCEALER 13 FUDGE
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:31720-965 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) TRISILOXANE (UNII: 9G1ZW13R0G) KAOLIN (UNII: 24H4NWX5CO) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) BORON NITRIDE (UNII: 2U4T60A6YD) TALC (UNII: 7SEV7J4R1U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEVOMENOL (UNII: 24WE03BX2T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCERIN (UNII: PDC6A3C0OX) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHAMOMILE (UNII: FGL3685T2X) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31720-965-18 1 in 1 BOX 06/01/2020 1 3.5 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/01/2020 Labeler - S+ (572406531)