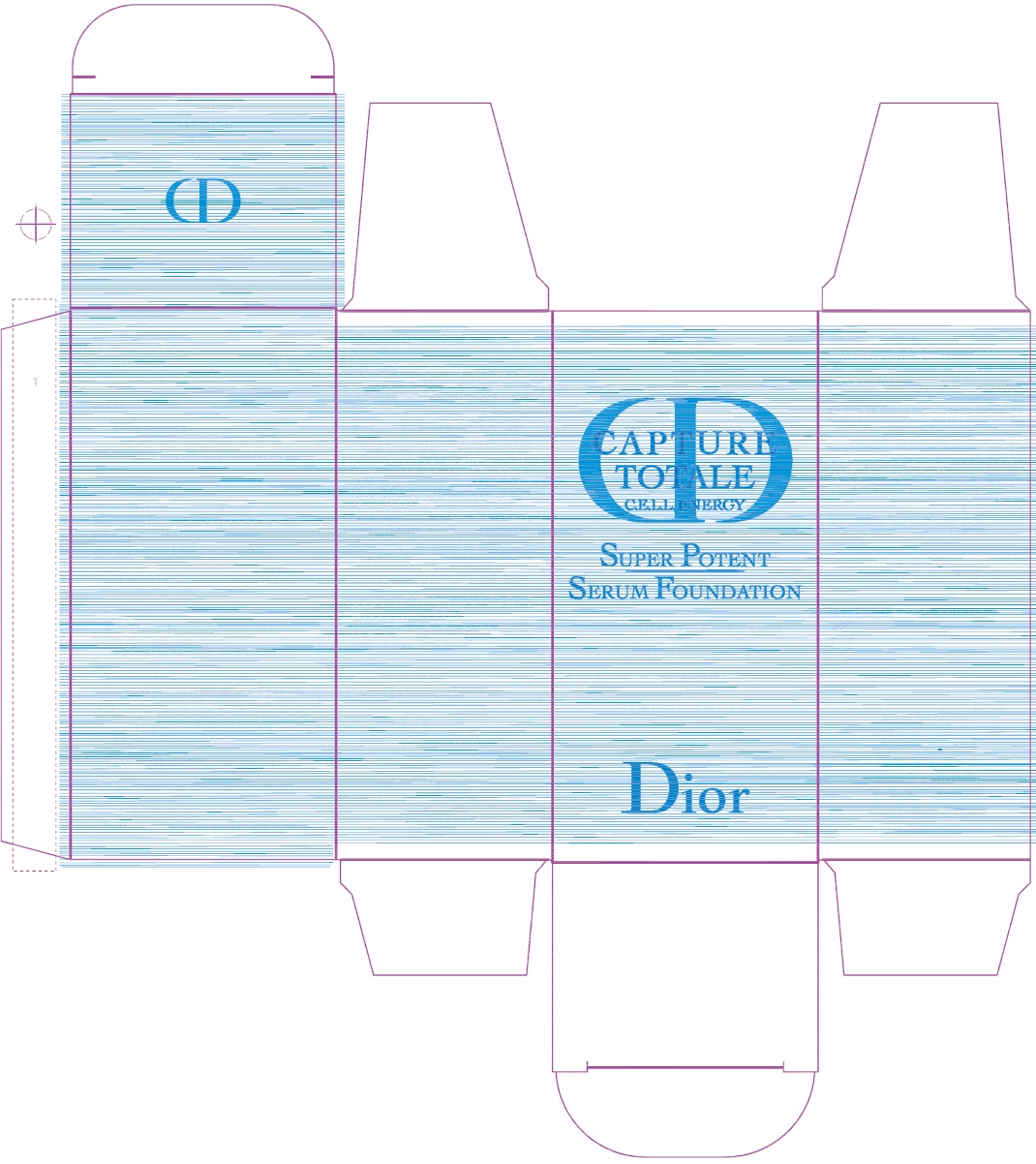
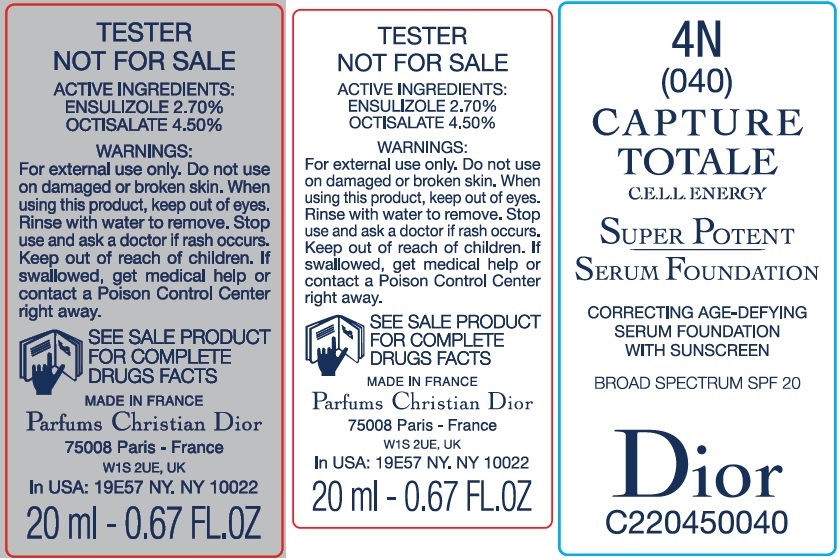
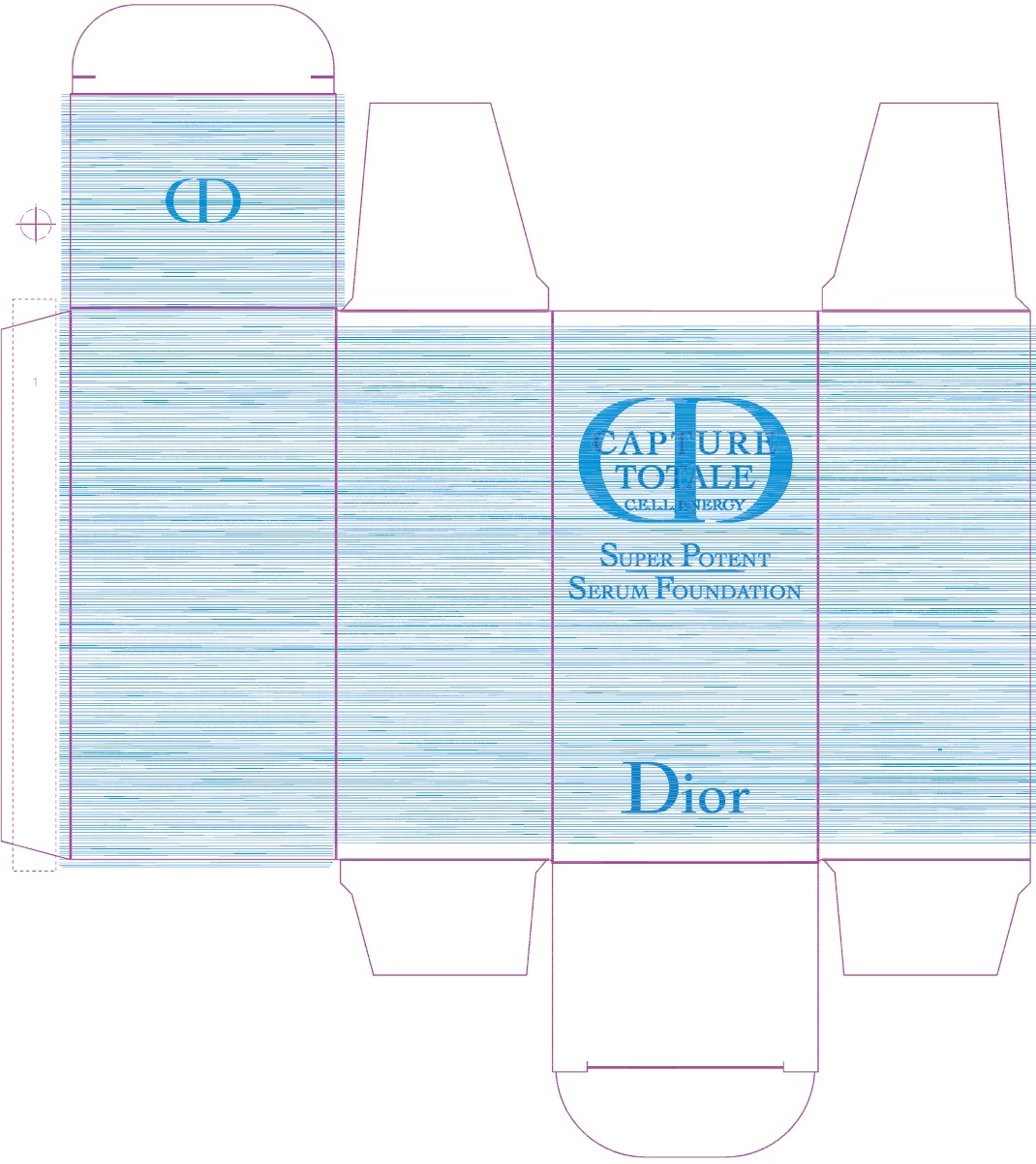
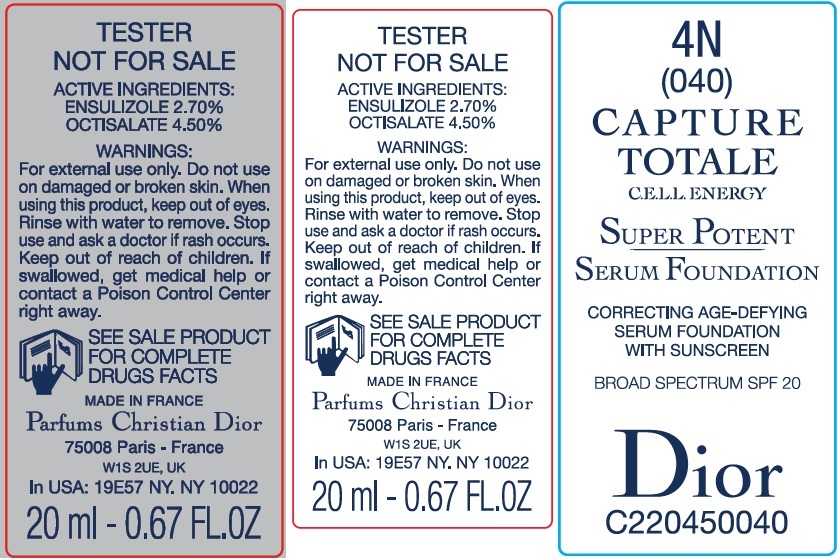
Label: CAPTURE TOTALE C.E.L.L. ENERGY SUPER POTENT SERUM FOUNDATION BROAD SPECTRUM SPF 20 4N- ensulizole, octisalate emulsion
- NDC Code(s): 61957-2407-0, 61957-2407-1
- Packager: Parfums Christian Dior
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Sun Protection Measures.
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses.
- Children under 6 months of age: ask a doctor.
- Other information
-
Inactive ingredients
• AQUA (WATER) • DIMETHICONE • METHYL TRIMETHICONE • LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE • PHENYL TRIMETHICONE • SYNTHETIC FLUOROPHLOGOPITE • GLYCERIN • PENTYLENE GLYCOL • SILICA • BUTYLENE GLYCOL• SORBITAN SESQUIOLEATE • TROMETHAMINE • ACRYLATES/DIMETHICONE COPOLYMER • MICA • DIMETHICONOL • PARFUM (FRAGRANCE) • SODIUM MYRISTOYL GLUTAMAT • GLYCERYL UNDECYL DIMETHICONE • CHLORPHENESIN • CAPRYLYL GLYCOL• SODIUM CHLORIDE • DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER • POLYSORBATE 80 • HYDROGEN DIMETHICONE • ALUMINUM HYDROXIDE • ADENOSINE• SODIUM ACETYLATED HYALURONATE • XYLITOL • TOCOPHEROL • SODIUM SURFACTIN • AFRAMOMUM ANGUSTIFOLIUM SEED EXTRACT • PALMARIA PALMATA EXTRACT • SODIUM BENZOATE • HYDROLYZED SOY PROTEIN • PAEONIA LACTIFLORA ROOT EXTRACT • TREHALOSE • POTASSIUM SORBATE • LILIUM CANDIDUM BULB EXTRACT • JASMINUM OFFICINALE (JASMINE) FLOWER EXTRACT. [MAY CONTAIN:CI 77491,CI 77492,CI 77499 (IRON OXIDES) • CI 77891 (TITANIUM DIOXIDE) • CI 77947 (ZINC OXIDE)].
N °15310/Z - Package Labeling:61957-2407-0
- Package Labeling:61957-2407-1
-
INGREDIENTS AND APPEARANCE
CAPTURE TOTALE C.E.L.L. ENERGY SUPER POTENT SERUM FOUNDATION BROAD SPECTRUM SPF 20 4N
ensulizole, octisalate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61957-2407 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 27 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) GLYCERIN (UNII: PDC6A3C0OX) PENTYLENE GLYCOL (UNII: 50C1307PZG) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) TROMETHAMINE (UNII: 023C2WHX2V) MICA (UNII: V8A1AW0880) DIMETHICONOL (100000 CST) (UNII: OSA9UP217S) SODIUM MYRISTOYL GLUTAMATE (UNII: AYU7QD893W) CHLORPHENESIN (UNII: I670DAL4SZ) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM CHLORIDE (UNII: 451W47IQ8X) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ADENOSINE (UNII: K72T3FS567) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) XYLITOL (UNII: VCQ006KQ1E) TOCOPHEROL (UNII: R0ZB2556P8) AFRAMOMUM ANGUSTIFOLIUM SEED (UNII: OSF83Q896J) PALMARIA PALMATA (UNII: 7832HOY4ZQ) SODIUM BENZOATE (UNII: OJ245FE5EU) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) PAEONIA LACTIFLORA ROOT (UNII: 3Z3866YW6P) TREHALOSE (UNII: B8WCK70T7I) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61957-2407-0 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2021 2 NDC:61957-2407-1 20 mL in 1 TUBE; Type 0: Not a Combination Product 09/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2021 Labeler - Parfums Christian Dior (275252245)