Label: VICKS SINEX SEVERE ORIGINAL- oxymetazoline hydrochloride spray
- NDC Code(s): 64336-170-01
- Packager: Procter & Gamble Manufacturing GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
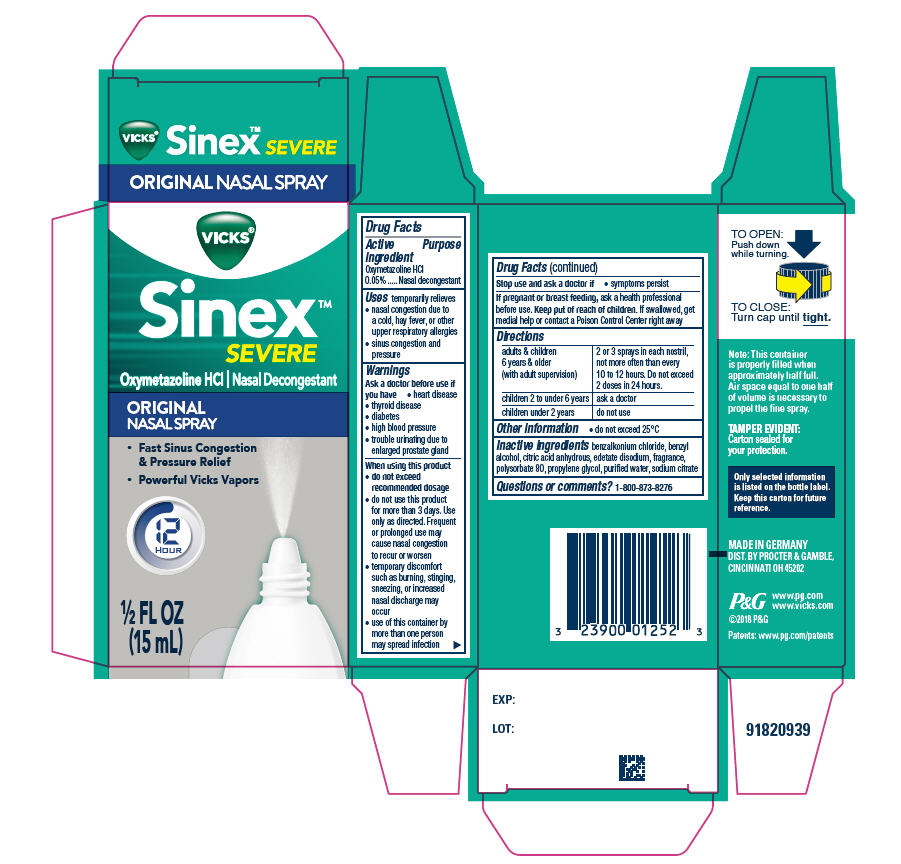
- Active ingredient
- Purpose
- Uses
- Warnings
-
WHEN USING
When using this product
- do not exceed recommended dosage
- do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen
- temporary discomfort such as burning, stinging, sneezing, or increased nasal discharge may occur
- use of this container by more than one person may spread infection
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- SPL UNCLASSIFIED SECTION
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 15 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
VICKS SINEX SEVERE ORIGINAL
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64336-170 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.0005095 g in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) Product Characteristics Color white (Clear) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64336-170-01 1 in 1 CARTON 12/20/2018 1 15 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/20/2018 Labeler - Procter & Gamble Manufacturing GmbH (333608813)