Label: DOLOEAR- apis melifica, aristolochia clematitis, belladonna, chamomilla, lachesis mutus, thuja occidentalis solution/ drops
- NDC Code(s): 55758-327-15
- Packager: Pharmadel LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Active ingredients & Purposes
Active Ingredients** (HPUS) Purposes* Apis melifica 6C ....................... Ear pain, inflammation
Aristolochia clematitis 6C......... Ear inflammation Belladonna 6C.......................... Throbbing pain Chamomilla vulgaris 6C .......... Ear pain irritability
Lachesis mutus 6C .................. Ear inflammation Thuja occidentalis 6C .............. Earache from pressure The letters HPUS indicate that this ingredient is officially included in the Homeopathic Pharmacopeia of the United States.
- Uses*
- Warnings
-
Directions
- shake before use
adults and children ages 6 and older:
- tilt head sideways and apply 2-3 drops into the ear (tip of dropper should not enter the ear canal)
- keep head tilted and allow drops to remain in the ear for 2 minutes or gently place cotton in ear to keep drops in
- repeat as needed, up to 4 times a day
children under 6 years of age: do not use
- Other Information
- Inactive Ingredients
- Distributed by:
- Homeopathic Statements
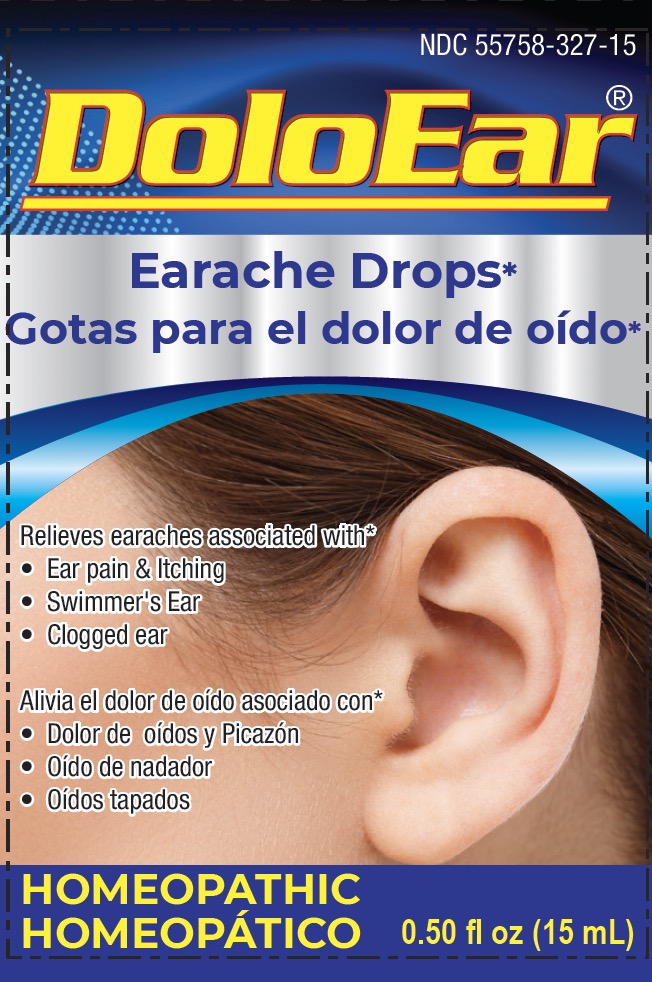
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DOLOEAR
apis melifica, aristolochia clematitis, belladonna, chamomilla, lachesis mutus, thuja occidentalis solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55758-327 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 6 [hp_C] in 15 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 6 [hp_C] in 15 mL ARISTOLOCHIA CLEMATITIS ROOT (UNII: ZY0NX0W00D) (ARISTOLOCHIA CLEMATITIS ROOT - UNII:ZY0NX0W00D) ARISTOLOCHIA CLEMATITIS ROOT 6 [hp_C] in 15 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 6 [hp_C] in 15 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 6 [hp_C] in 15 mL MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 6 [hp_C] in 15 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55758-327-15 1 in 1 BOX 09/07/2023 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2013 Labeler - Pharmadel LLC (030129680)