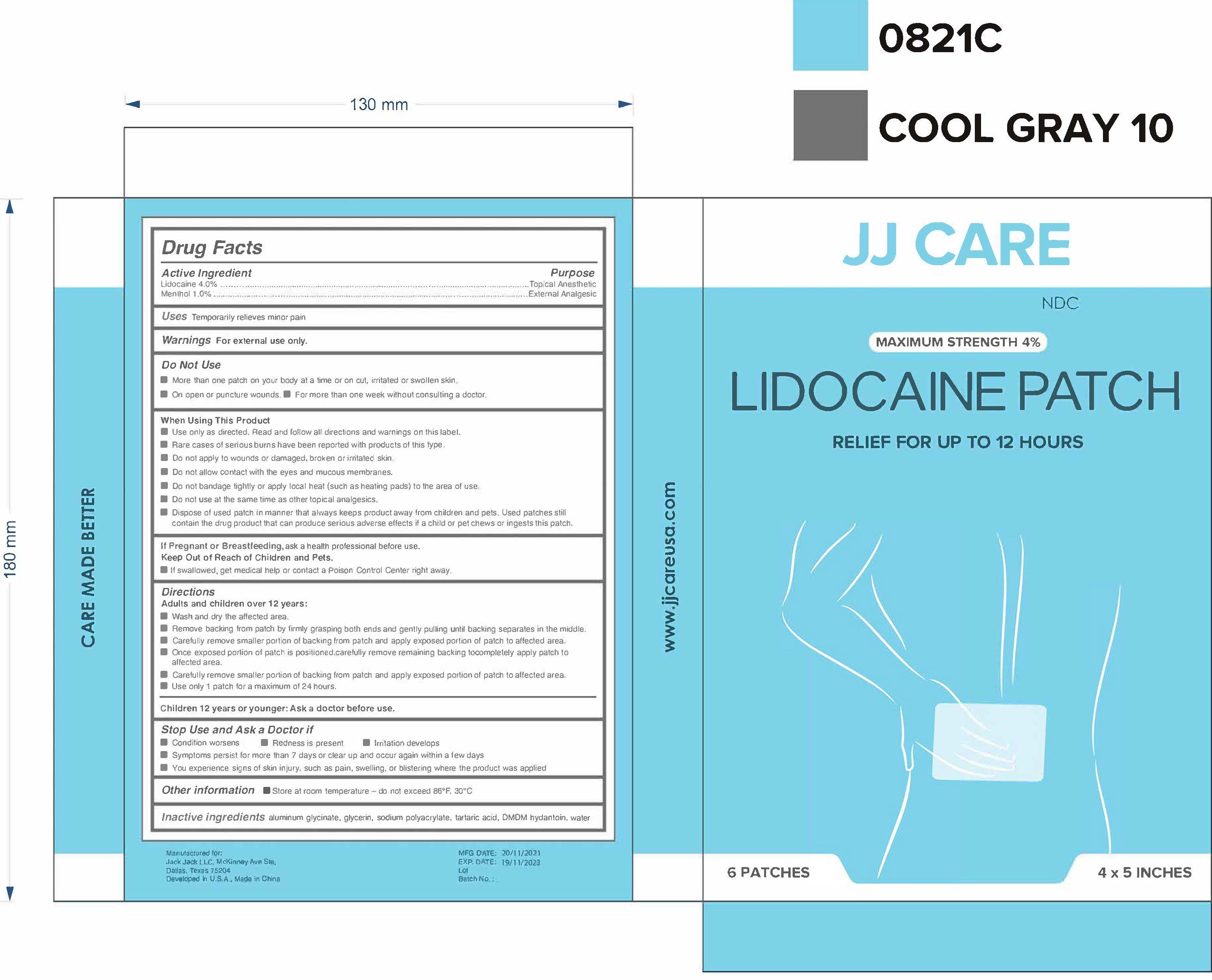
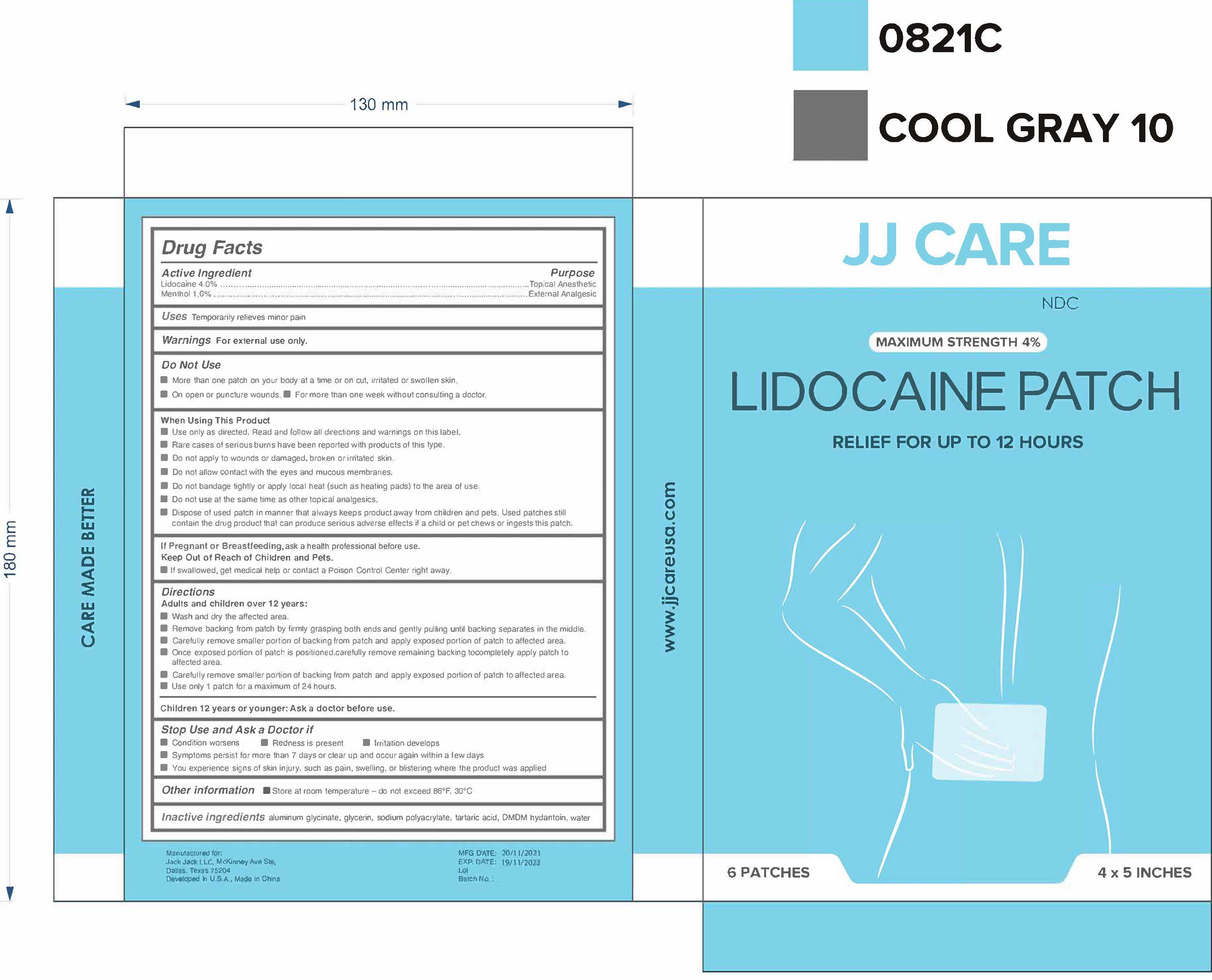
Label: LIDOCAINE PATCH- lidocaine 4% menthol 1% patch
- NDC Code(s): 81484-301-01, 81484-301-02
- Packager: Anhui Miao De Tang Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
-
WHEN USING
• Use only as directed. Read and邸ow all directions and warnings on this label.
• Rare cases of serious burns have been reported with products of this type.
• Oo not apply to wou心s or damaged, broken or irritated skin
• Do not allow contact with the eyes and mucous membranes.
• Do not bandage tightly or apply local heat (such as heating pads) to the area of use
• Do not use at the same time as other topical analgesics.
• Dispose of used patch in manner that always keeps producl away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Adults and children over 12 years:
• Wash and dry the affected area.
• Remove backing from patch by firmty grasping both ends and gently pulling until backing separates in the middle
• Carefully remove smaller portion of back,ng from patch and apply exposed portion of patch to a什ected area
• Once exposed portion of patch is po奾oned.carefully remove remaining backing tocomplelely apply patch to affected area.
• Carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
• Use only 1 patch for a maximum of 24 hours.
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
LIDOCAINE PATCH
lidocaine 4% menthol 1% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81484-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.1 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.4 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) DMDM HYDANTOIN (UNII: BYR0546TOW) TARTARIC ACID (UNII: W4888I119H) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81484-301-02 6 in 1 BOX 11/01/2021 1 NDC:81484-301-01 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2021 Labeler - Anhui Miao De Tang Pharmaceutical Co., Ltd. (405744102) Establishment Name Address ID/FEI Business Operations Anhui Miao De Tang Pharmaceutical Co., Ltd. 405744102 manufacture(81484-301)