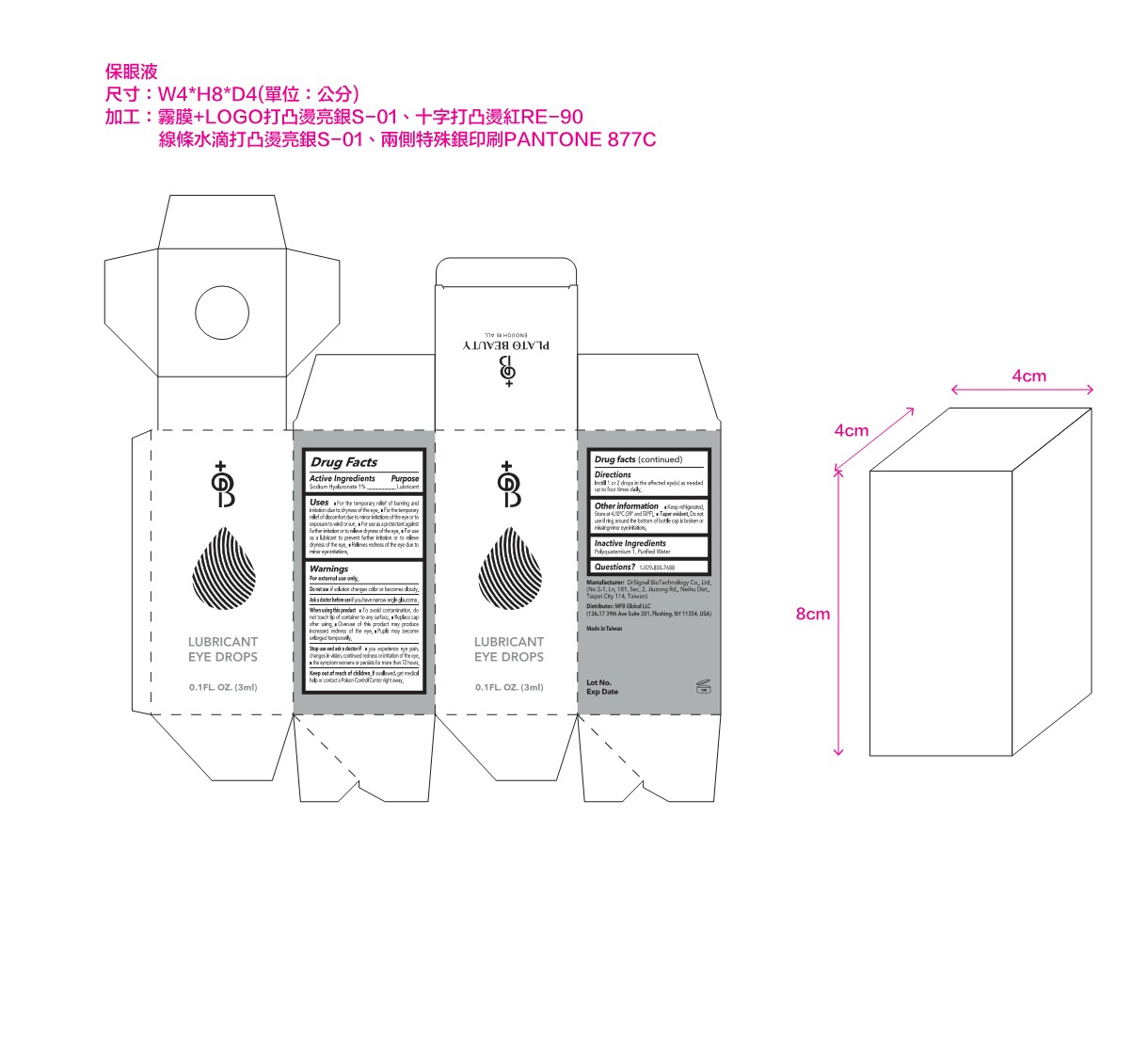
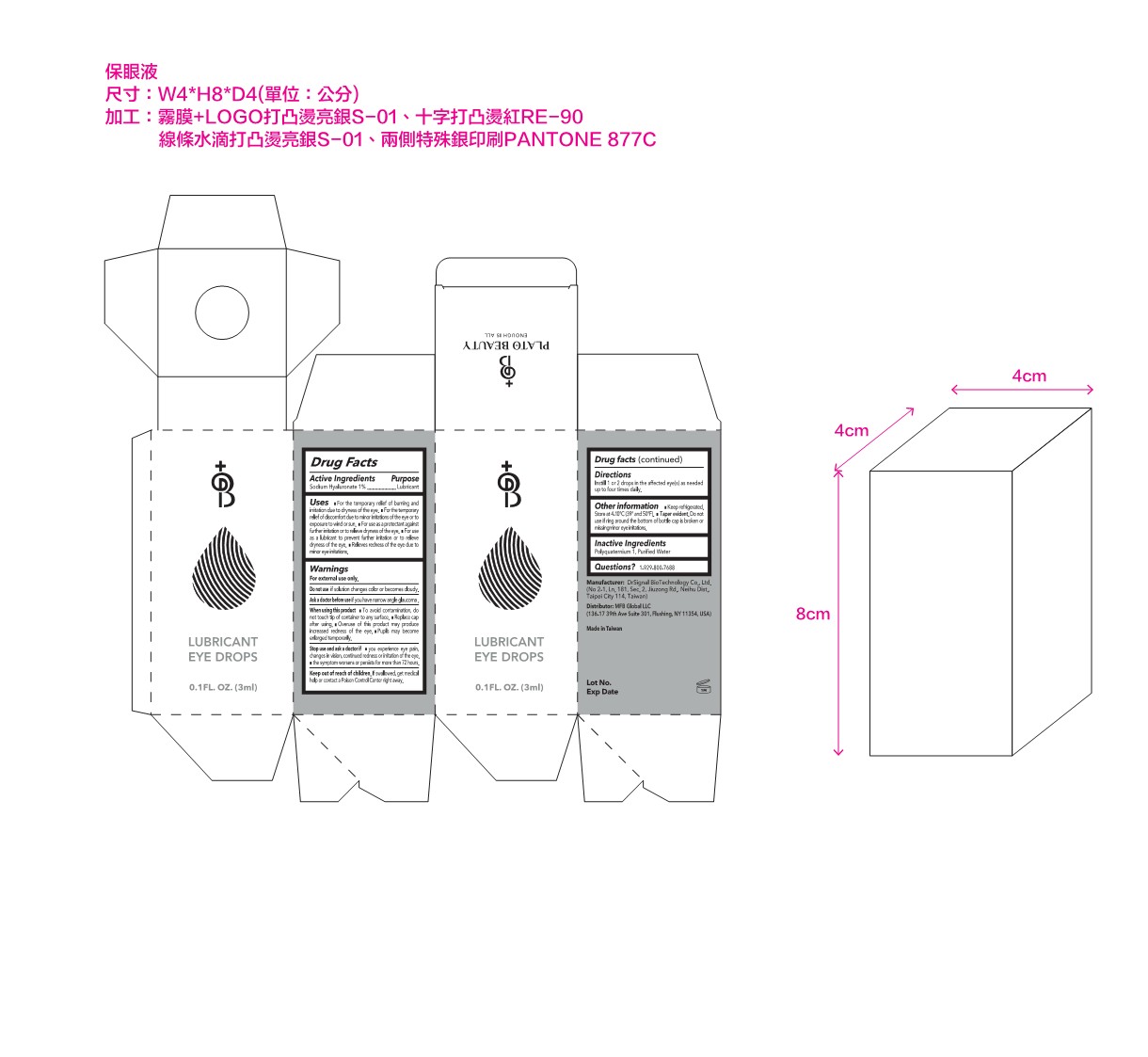
Label: LUBRICANT EYE DROPS- sodium hyaluronate solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 82357-1101-1, 82357-1101-2 - Packager: MFB Global LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 7, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
DOSAGE & ADMINISTRATION
For the temporary relief of burning and irritation due to dryness of the eye.
For the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun.
For use as a protectant against further irritation or to relieve dryness of the eye.
For use as a lubricant to prevent further irritation or to relieve dryness of the eye.
Relieves redness of the eye due to minor eye irritations.
- WARNINGS
- DO NOT USE
- USER SAFETY WARNINGS
- WHEN USING
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- 88436-1 - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LUBRICANT EYE DROPS
sodium hyaluronate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82357-1101 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONATE SODIUM 3 g in 100 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 0.3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLIDRONIUM CHLORIDE (UNII: 6716Z5YR3G) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82357-1101-2 1 in 1 BOX 11/05/2021 1 NDC:82357-1101-1 3 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/05/2021 Labeler - MFB Global LLC (118369923) Registrant - DrSignal BioTechnology Co., Ltd. (656113584) Establishment Name Address ID/FEI Business Operations DrSignal BioTechnology Co., Ltd. 656113584 manufacture(82357-1101)