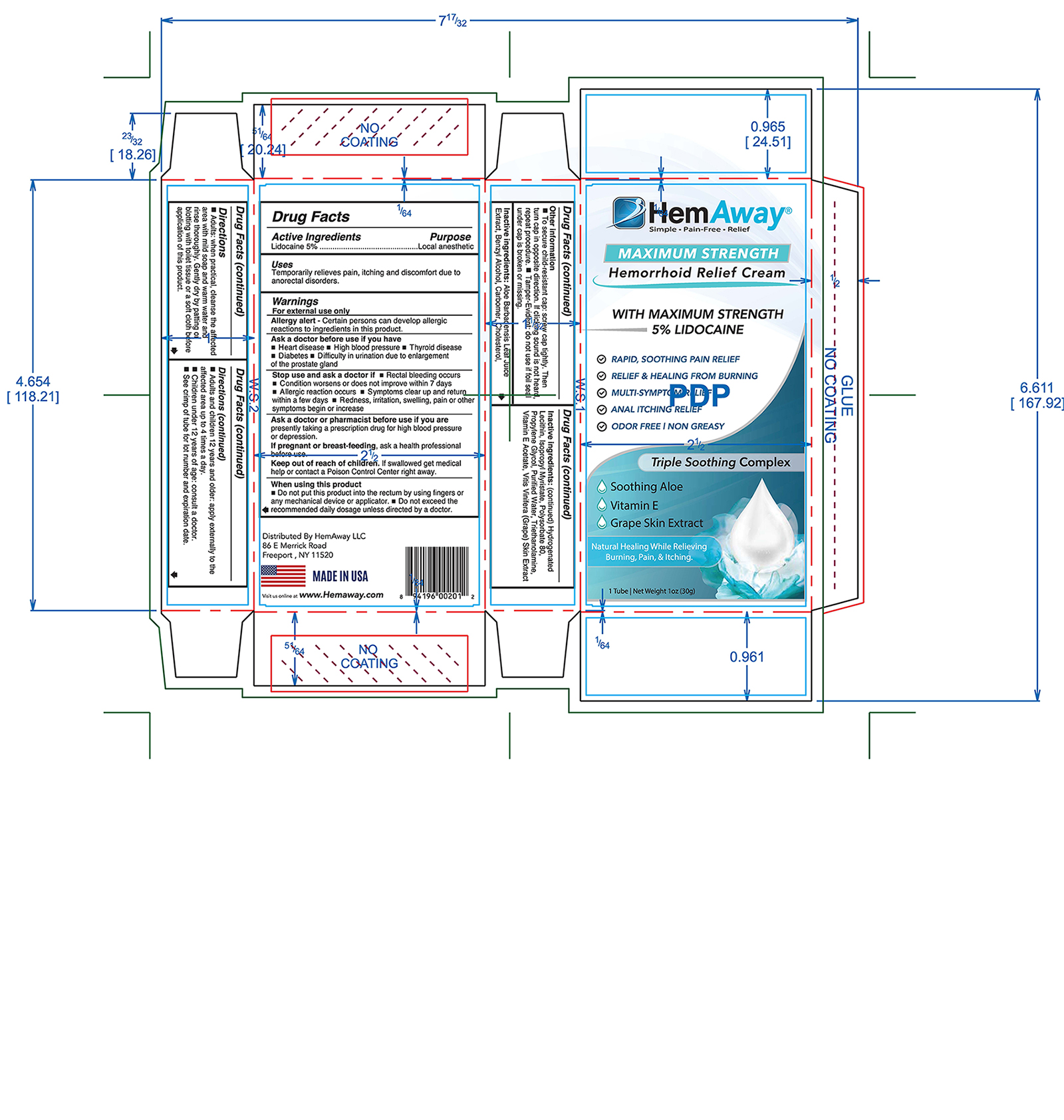
Label: HEMAWAY- lidocaine cream
- NDC Code(s): 54473-336-01, 54473-336-30
- Packager: Melaleuca, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
- Adults: when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
- Adults and children 12 years and older: apply externally to the affected area up to 4 times a day.
- Children under 12 years of age: consult a doctor.
- QUESTIONS
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- ASK DOCTOR
- STOP USE
- ASK DOCTOR/PHARMACIST
- PREGNANCY OR BREAST FEEDING
- WHEN USING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMAWAY
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54473-336 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 1.5 g in 30 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) CHOLESTEROL (UNII: 97C5T2UQ7J) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GRAPE (UNII: 6X543N684K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54473-336-01 1 in 1 BOX 08/04/2020 1 NDC:54473-336-30 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 08/04/2020 Labeler - Melaleuca, Inc. (139760102) Establishment Name Address ID/FEI Business Operations Melaleuca, Inc. 805617610 manufacture(54473-336)