Label: DI-METHOX- sulfadimethoxine injection
- NDC Code(s): 23243-0410-5
- Packager: Huvepharma, Inc
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated May 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- GENERAL PRECAUTIONS
-
INDICATIONS & USAGE
INDICATIONS: CATTLE – For the treatment of bovine respiratory disease complex
(shipping fever complex) and bacterial pneumonia associated with Pasteurella
spp. sensitive to sulfadimethoxine; necrotic pododermatitis (foot rot) and
calf diphtheria caused by Fusobacterium necrophorum (Sphaerophorus necrophorus),
sensitive to sulfadimethoxine. -
RESIDUE WARNING
RESIDUE WARNINGS: Milk taken from animals during treatment and for 60 hours (5 milkings)
after the latest treatment must not be used for food.Do not administer within 5 days of slaughter. A withdrawal period has not been established
for this product in pre-ruminating calves. Do not use in calves to be processed for veal.LOT NO.: EXP. DATE:
-
DOSAGE & ADMINISTRATION
EACH mL CONTAINS: 400 mg sulfadimethoxine compounded with 20% propylene glycol, 1% benzyl alcohol, 0.1 mg
disodium edetate, 1 mg sodium formaldehyde sulfoxylate, and pH adjusted with sodium hydroxide.DOSAGE: To be administered in amounts to provide 25 mg/lb (55 mg/kg) for initial dose, followed by
12.5 mg/lb (27.5 mg/kg) for maintenance doses every 24 hours.During treatment period, make certain that animals maintain adequate water intake.
For detailed dosage recommendations, indications, limitations and precautions, read accompanying booklet.
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- GENERAL PRECAUTIONS
- USER SAFETY WARNINGS
-
DESCRIPTION
DESCRIPTION: DI-METHOX® Injection 40% is a low-dosage, rapidly
absorbed, long-acting sulfonamide, effective for the treatment of
shipping fever complex, bacterial pneumonia, calf diphtheria and foot
rot in cattle. Sulfadimethoxine is a white, almost tasteless and odorless
compound. Chemically, it is N1-(2,6 dimethoxy-4-pyrimidinyl)
sulfanilamide. The structural formula is: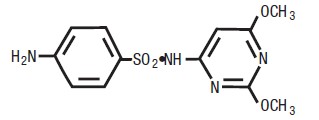
-
MECHANISM OF ACTION
ACTIONS: Sulfadimethoxine has been demonstrated clinically or in the laboratory to be effective against
a variety of organisms, such as streptococci, klebsiella, proteus, shigella, staphylococci, escherichia,
and salmonella.1,2 The systemic sulfonamides which include sulfadimethoxine are bacteriostatic agents.
Sulfonamides competitively inhibit bacterial synthesis of folic acid (pteroylglutamic acid) from
para-aminobenzoic acid. Mammalian cells are capable of utilizing folic acid in the presence of sulfonamides.The tissue distribution of sulfadimethoxine, as with all sulfonamides, is a function of plasma levels,
degree of plasma protein binding, and subsequent passive distribution in the tissues of the lipid-soluble
un-ionized form. The relative amounts are determined by both its pKa and by the pH of each tissue.
Therefore, levels tend to be higher in less acid tissue and body fluids or those diseased tissues having
high concentrations of leucocytes.2
Slow renal excretion results from a high degree of tubular reabsorption,3 and plasma protein binding
is very high, providing a blood reservoir of the drug. Thus, sulfadimethoxine maintains higher blood levels
than most other long-acting sulfonamides. Single, comparatively low doses of sulfadimethoxine give rapid and
sustained therapeutic blood levels.1To assure successful sulfonamide therapy (1) the drug must be given early in the course of the disease,
and it must produce a high sulfonamide level in the body rapidly after administration, (2) therapeutically
effective sulfonamide levels must be maintained in the body throughout the treatment period, (3) treatment
should continue for a short period of time after the clinical signs have disappeared, and (4) the causative
organisms must be sensitive to this class of drugs. -
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
TOXICITY AND SAFETY: Data regarding acute (LD50) and chronic toxicities of sulfadimethoxine indicate the drug is very safe. The LD50 in mice is greater than 2 g/kg body weight when administered intraperitoneally and greater than 16 g/kg when administered orally.
In dogs receiving massive single oral doses of 3.2 g/kg body weight, diarrhea was the only adverse effect observed. Dogs given 160 mg/kg body weight orally daily for 13 weeks showed no signs of toxicity.
In cattle sulfadimethoxine has been shown to be safe through extensive clinical use with other dosage forms. In addition, studies with intravenous administration of DI-METHOX® Injection 40% have demonstrated that hemolysis of erythrocytes does not occur by this route of administration. Sulfadimethoxine has a relatively high solubility at the pH normally occurring in the kidney, precluding the possibility of precipitation and crystalluria.
-
INDICATIONS & USAGE
INDICATIONS: DI-METHOX® Injection 40% is indicated for the treatment
of bovine respiratory disease complex (shipping fever complex) and
bacterial pneumonia associated with Pasteurella spp. sensitive to
sulfadimethoxine; necrotic pododermatis (foot rot) and calf diphtheria
caused by Fusobacterium necrophorum (Sphaerophorus necrophorus),
sensitive to sulfadimethoxine.
LIMITATIONS: Sulfadimethoxine is not effective in viral or rickettsial
infections, and as with any anti-bacterial agent, occasional failures in
therapy may occur due to resistant microorganisms. The usual
precautions in sulfonamide therapy should be observed. -
RESIDUE WARNING
RESIDUE WARNINGS: Milk taken from animals during treatment and for 60 hours (5 milkings) after the latest
treatment must not be used for food. Do not administer within 5 days of slaughter. A withdrawal period has not been
established for this product in pre-ruminating calves. Do not use in calves to be processed for veal. - PRECAUTIONS
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: DI-METHOX® Injection 40% must be
administered only by the intravenous route in cattle. Cattle should
receive 1 mL of DI-METHOX® Injection 40% per 16 pounds of body
weight (55 mg/kg) as an initial dose, followed by 0.5 mL per 16 pounds
of body weight (27.5 mg/kg) every 24 hours thereafter. Sulfadimethoxine
boluses may be utilized for maintenance therapy in cattle.
Representative weights and doses are indicated in the following table: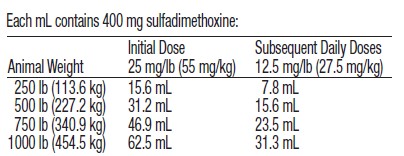
Length of treatment depends on the clinical response. In most cases
treatment for 3 to 5 days is adequate. Treatment should be continued
until the animal is asymptomatic for 48 hours. -
INSTRUCTIONS FOR USE
DIRECTIONS FOR INTRAVENOUS INJECTION:
Equipment needed –
1. A nose lead and/or halter sufficiently strong enough to effectively restrain or hold the animal's head steady so that the intravenous
injection can be made with ease.
2. Hypodermic needles, 16 or 18 gauge and 2 inches long. Only new, sharp and sterile hypodermic needles should be used.
Dull needles should be discarded. Extra needles should always be available in case the needle being used should become clogged.
3. Hypodermic syringes, 40 or 50 mL sterile disposable or reusable glass syringes should be available.
4. Alcohol (70%) or equally effective antiseptic for disinfecting the skin.
Preparation of equipment – Glass syringes and regular hypodermic needles should be thoroughly cleaned and washed. Following this, the needles and syringes should be immersed in boiling water for 30 minutes prior to each injection. Regular hypodermic needles should not be used more than 3-4 times as repeated skin puncturing and boiling of the needles causes them to become quite dull. Disposable hypodermic needles and syringes should not be used more than once.
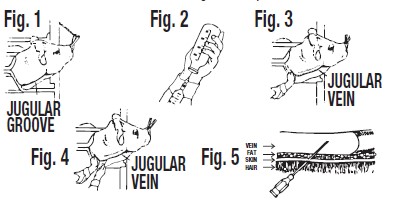
Restraint of animal – The cow should preferably be in a stanchion for maximum restraint. If this is not possible, the animal should be restrained in a manner to prevent excessive movement. A nose lead should be applied and the animal's head turned sidewise to stretch the skin and tense the muscles of the neck region. (See Figure 1).
Locating the jugular vein – Once the animal has been restrained (as above), you will notice a long depression of the skin from below the angle of the jaw to just above the shoulder. This is known as the jugular furrow or jugular groove. The jugular vein is located just under the jugular groove. (See Figure 1).
Preparation of DI-METHOX® Injection 40% for injection – The rubber cap of the bottle should be thoroughly cleaned with 70% alcohol or other satisfactory antiseptic. The correct amount of DI-METHOX® Injection 40% for treatment should be calculated (see dosage directions) and that amount withdrawn into a syringe. (See Figure 2). One or two syringefuls of air should be injected into the bottle first to make withdrawing the drug easier. DI-METHOX® Injection 40% should preferably be at room temperature when filling syringes and when injecting intravenously.
Entering the vein – The skin of the injection area should be clean and free of dirt. Cotton saturated with 70% alcohol (or suitable antiseptic) should be used to wipe the injection site.
Apply pressure over the jugular vein close to the shoulder. This will reduce the flow of blood to the heart and cause the jugular vein to bulge or enlarge. (See Figure 3). When the jugular vein has been "raised", insert the hypodermic needle at a 45 degree angle through the skin just underneath the jugular vein. The beveled edge of the hypodermic needle should be up. (See Figure 4).
After the skin has been punctured, the point of the needle should be directed toward the side of the vein and pushed into the center of the vein. (See Figure 5). When the needle is in the center of the vein, there will be a free flow of blood back through the needle. Release external pressure when you are sure the needle is within the vein.
Injecting the DI-METHOX® Injection 40% – After the needle has been accurately inserted into the jugular vein, firmly attach the syringe containing DI-METHOX® Injection 40% to the inserted hypodermic needle. Caution, be sure syringe is free of air. Exert firm pressure on the plunger of the syringe to inject the DI-METHOX® Injection 40% while the barrel is held firmly. The injection should be made moderately slow – never rapidly.
If the animal moves, causing resistance in pushing the plunger of the syringe, or if a bubble of the drug is noted under the skin, the needle is no longer within the vein. The needle should be repositioned.When the injection is completed, quickly withdraw the syringe and needle with a quick pull and apply light pressure over the injection site with alcohol and cotton to minimize bleeding from the puncture site.
-
ADVERSE REACTIONS
To report suspected adverse drug events, for technical assistance or to
obtain a copy of the Safety Data Sheet (SDS), contact Huvepharma, Inc. at
1-877-994-4883 or www.huvepharma.us. For additional information
about adverse drug experience reporting for animal drugs, contact FDA at
1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
NOTE: Store at room temperature. Should crystallization occur at cold
temperatures, crystals will dissolve either by storing at room temperature
for several days or by heating the vial in warm water. Crystallization and
redissolution do not impair the efficacy of the product. -
HOW SUPPLIED
HOW SUPPLIED: DI-METHOX® Injection 40% is available in
250 mL sterile multiple dose containers.
DI-METHOX® Injection 40% – Each mL contains 400 mg
sulfadimethoxine compounded with 20% propylene glycol,
1% benzyl alcohol, 0.1 mg disodium edetate, 1 mg sodium
formaldehyde sulfoxylate, and pH adjusted with sodium hydroxide. -
REFERENCES
REFERENCES
1. Data on file from Hoffmann-La Roche Inc., Nutley, New Jersey.
2. Stowe, C.M., THE SULFONAMIDES, in Jones. L.M. (ed.),
Veterinary Pharmacology and Therapeutics, Ames, Iowa, Iowa
State University Press. 1965, chapter 33.
3. Baggot, J.D., SOME ASPECTS OF DRUG PERSISTENCE IN
DOMESTIC ANIMALS, Res. Vet. Sci. 11:2, 130, 1970. -
SPL UNCLASSIFIED SECTION
® Registered trademark of Huvepharma, Inc.
Approved by FDA under ANADA # 200-038
Manufactured for
Huvepharma, Inc.
Peachtree City, GA 30269MF#S-4041-05
Rev. 05-2022EACH mL CONTAINS: 400 mg sulfadimethoxine compounded with 20%
propylene glycol, 1% benzyl alcohol, 0.1 mg disodium edetate, 1 mg
sodium formaldehyde sulfoxylate, and pH adjusted with sodium
hydroxide. - DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DI-METHOX
sulfadimethoxine injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:23243-0410 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFADIMETHOXINE (UNII: 30CPC5LDEX) (SULFADIMETHOXINE - UNII:30CPC5LDEX) SULFADIMETHOXINE 400 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM FORMALDEHYDE SULFOXYLATE (UNII: X4ZGP7K714) SODIUM HYDROXIDE (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23243-0410-5 250 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200038 04/15/2008 Labeler - Huvepharma, Inc (619153559)






