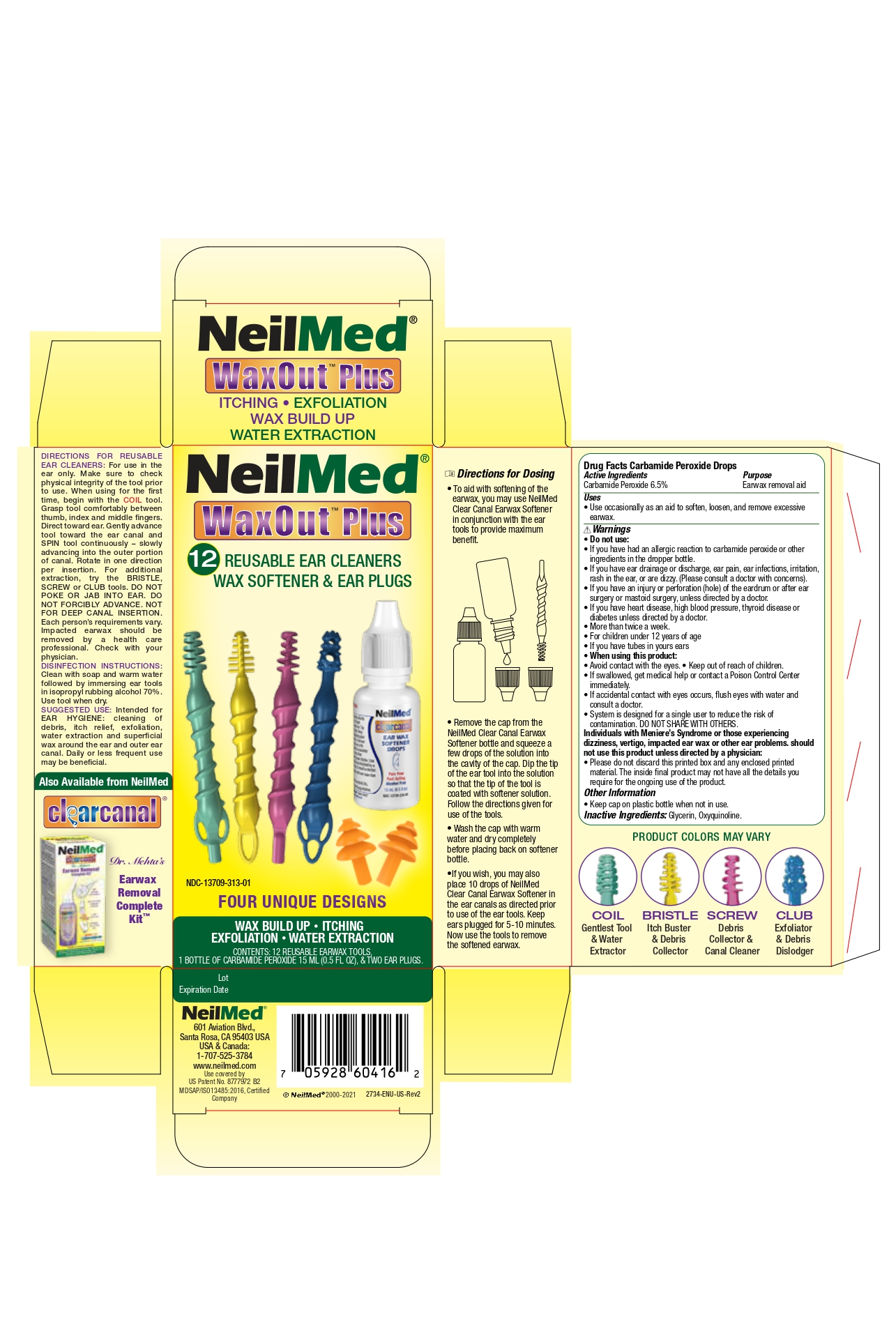
Label: WAX OUT PLUS- carbamide peroxide drops solution/ drops
- NDC Code(s): 13709-313-01
- Packager: NEILMED PHARMACEUTICALS INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Carbamide Peroxide drops Active Ingredients
- Purpose
- Uses
-
Warnings
Do not use:
- If you have had an allergic reactionto carbamide peroxide or other ingredients in teh dropper bottle.
- If you have ear drainage or discharge, ear pain, ear infections, irritation, rash in the ear, or are dizzy. (Please consult a doctor with concerns).
- If you have an injury or perforation (hole) of the eardrum or after ear surgery or mastoid surgery, unless directed by a doctor.
- More than a twice a week.
- For children under 12 years of age.
- If you have tubes in your ears.
-
When using this product:
- Avoid contact with eyes
- Keep out of reach of children
- If swallowed, get medical help or contact a poison control center immediately.
- If accidental contact with eyes occurs, flush eyes with water and consult a doctor.
- System is designed for a single user to reduce the risk of contamination. DO NOT SHARE WITH OTHERS
- Individuals with Meniere's Syndrome or those experiencing dizziness, vertigo, impacted earwax or other ear problems should not use this product unless directed by a physician.
- Please do not discard this printed box and any enclosed printed material. The inside final product may not have all the details you require for the ongoing use of the product.
- Other Information
- Inactive Ingredients
-
Directions for ReUsable Ear Cleaners
For use in the ear only. Make sure to check physical integrity of the tool prior to use. When usingfor teh first time, begin with the COIL tool. Grasp tool comfortably between thumb, index and middle fingers. Direct toward ear. Gently advance tool toward the earcanal and SPIN tool continuously - slowly advancing into the outer portion of canal. Rotate in one direction per insertion. For additional extraction, try the BRISTLE, SCREW or CLUB tools. DO NOT POKE OR JAB INTO EAR. DO NOT FORCIBLY ADVANCE. NOT FOR DEEP CANAL INSERTION. Each person's requirements vary. Impacted earwax should be removed by a health care professional. Check with your physician.
DISINFECTIONS INSTRUCTIONS:
Clean with soap and warm water followed by immersing eartools in isopropyl rubbing alcohol 70%. Use tool when dry.
SUGGESTED USE:
Intended for EAR HYGIENE: Cleaning of debris, itch relief, exfoliation, water extraction and superficial wax around the ear and outer ear canal. Daily or less frequent use may be beneficial.
-
Directions for Dosing
- To aid with the softening of teh earwax, you may use NeilMed Clear Canal Earwax Softener in conjunction with the eartools to provide maiximum benefit.
- Remove the cap from the NeilMed Clear Canal EarWax Softener bottle and squuze a few drops of the solution into the cavity of the cap. Dip the tip of the ear tool into the solution so that the tip of the tool is coated with softener solution. Follow the directions given for use of the tools.
- Wash the cap with warm water and dry completely before placing back on softener bottle
- If you wish, you may also place 10 drops of NeilMed Claer Canal Earwax Softener in teh earcanals as directed prior to use of the eartools. Keep ear plugged for 5-10 minutes. Now use the tools to remove the softened earwax.
- when Using this product
-
Warnings :
Do not use:
If you have had an allergic reactionto carbamide peroxide or other ingredients in teh dropper bottle.
If you have ear drainage or discharge, ear pain, ear infections, irritation, rash in the ear, or are dizzy. (Please consult a doctor with concerns).
If you have an injury or perforation (hole) of the eardrum or after ear surgery or mastoid surgery, unless directed by a doctor.
More than a twice a week.
For children under 12 years of age.
If you have tubes in your ears. - WaxOut PLus - Box Label
-
INGREDIENTS AND APPEARANCE
WAX OUT PLUS
carbamide peroxide drops solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13709-313 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 65 mg in 1 mL Inactive Ingredients Ingredient Name Strength OXYQUINOLINE (UNII: 5UTX5635HP) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13709-313-01 1 in 1 KIT 10/01/2019 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 11/05/2018 Labeler - NEILMED PHARMACEUTICALS INC (799295915) Establishment Name Address ID/FEI Business Operations NEILMED PHARMACEUTICALS INC 799295915 manufacture(13709-313)