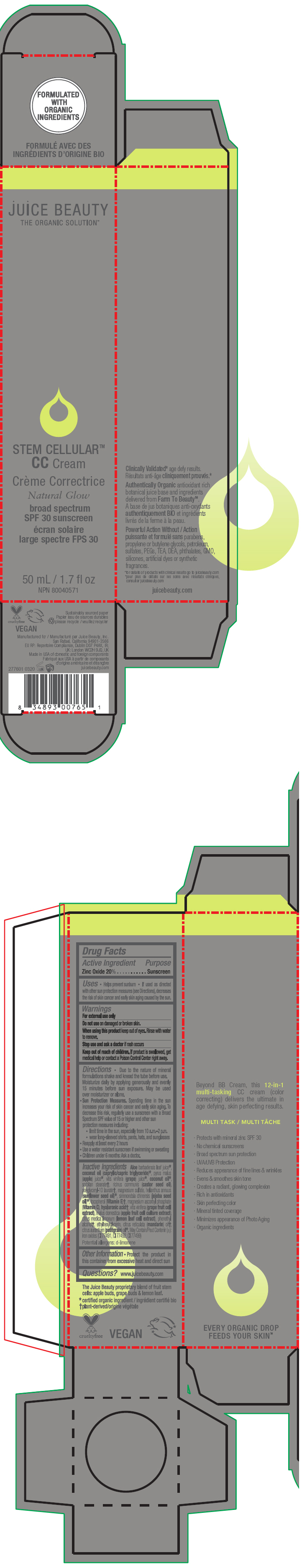
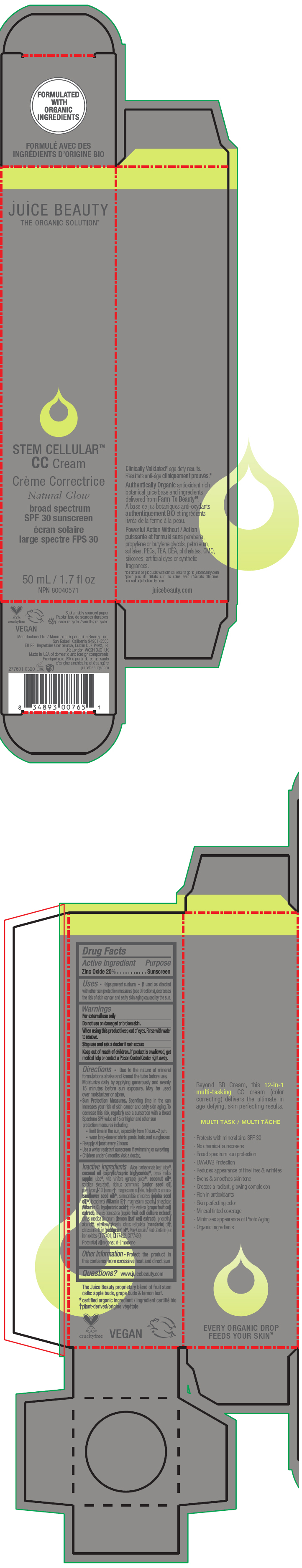
Label: STEM CELLULAR CC CREAM NATURAL GLOW- zinc oxide lotion
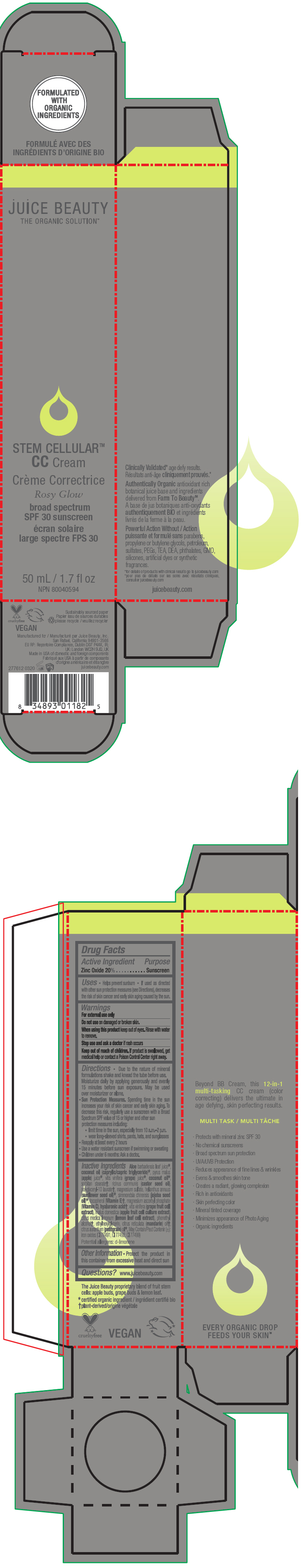
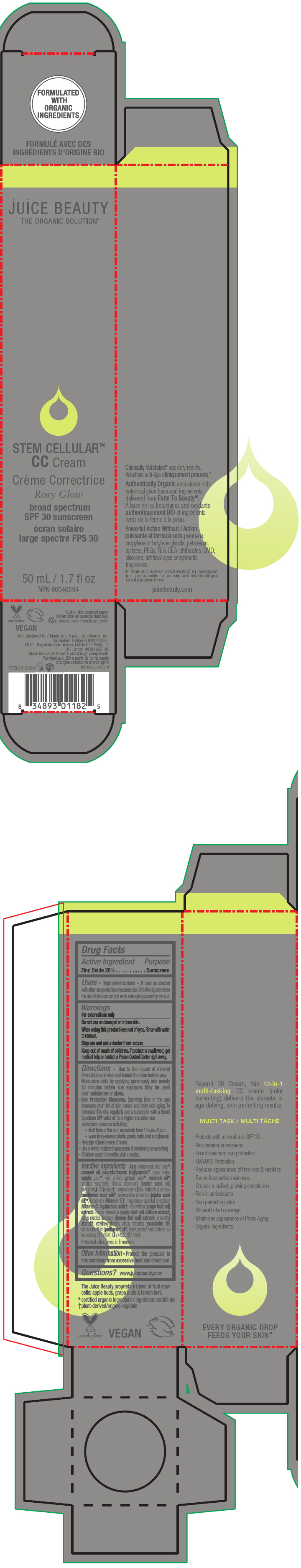
STEM CELLULAR CC CREAM ROSY GLOW- zinc oxide lotion
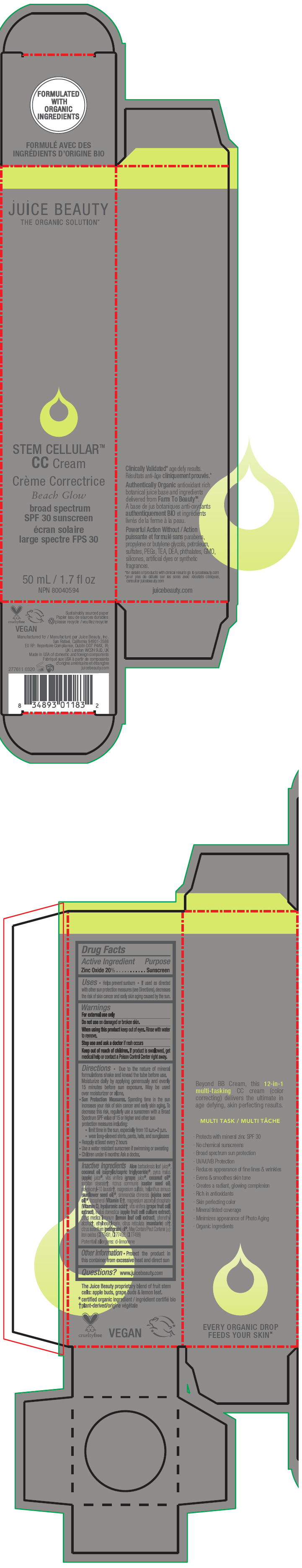
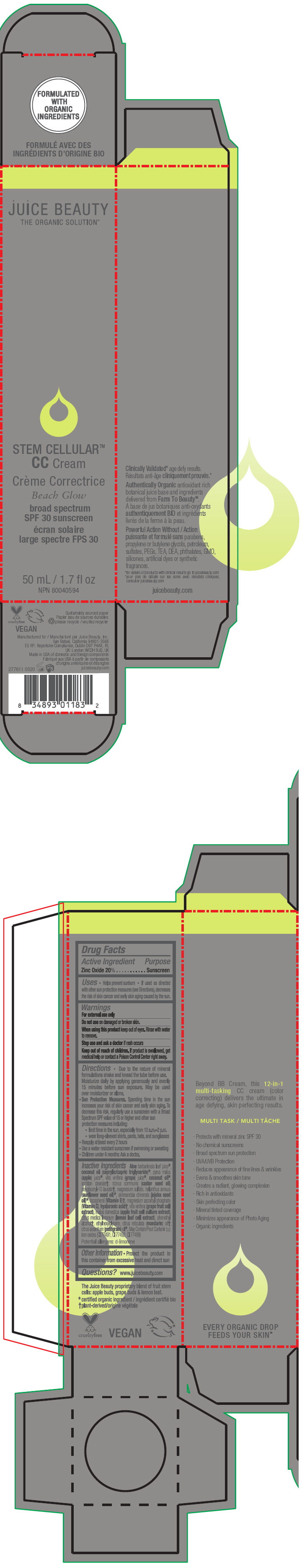
STEM CELLULAR CC CREAM BEACH GLOW- zinc oxide lotion
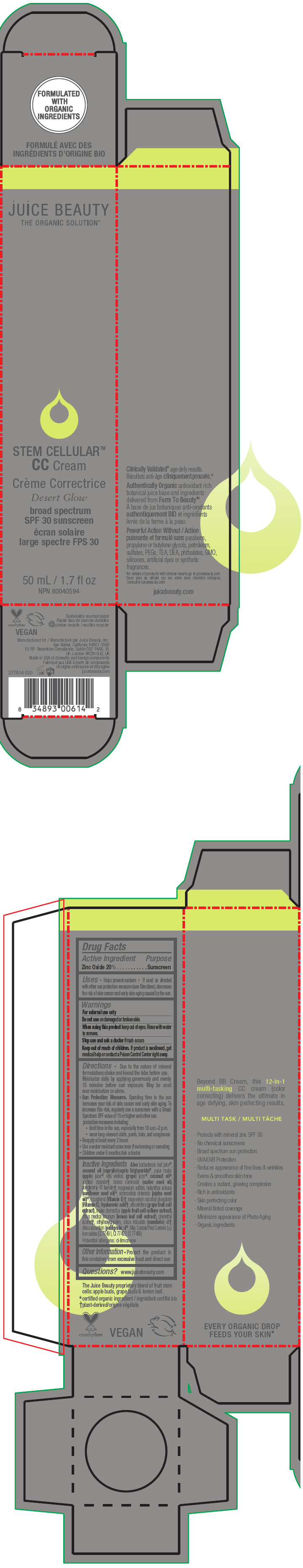
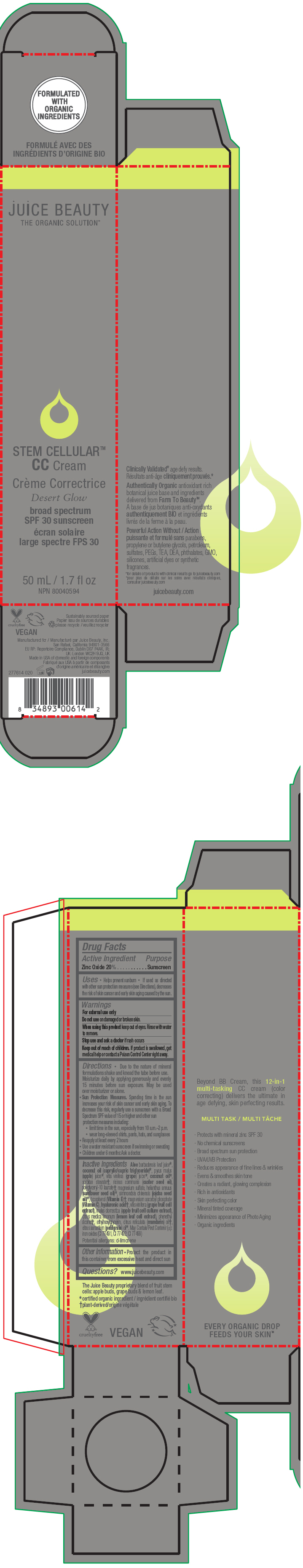
STEM CELLULAR CC CREAM DESERT GLOW- zinc oxide lotion
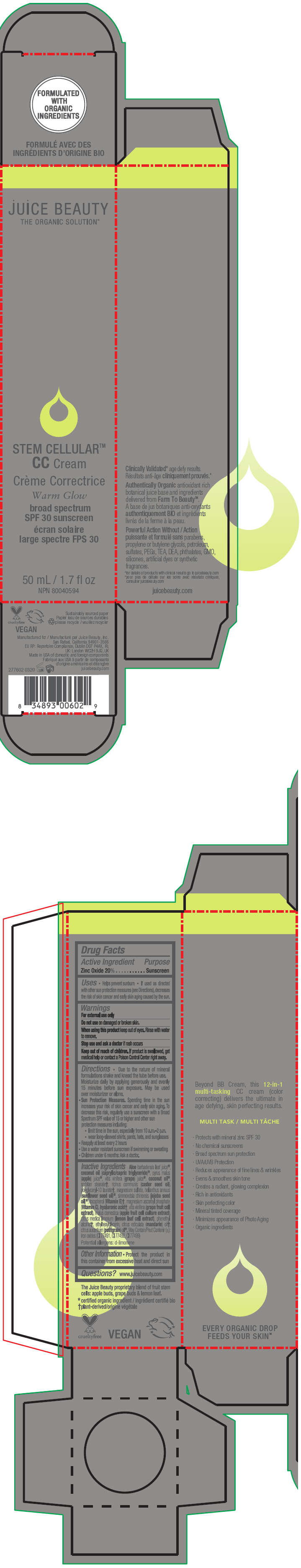
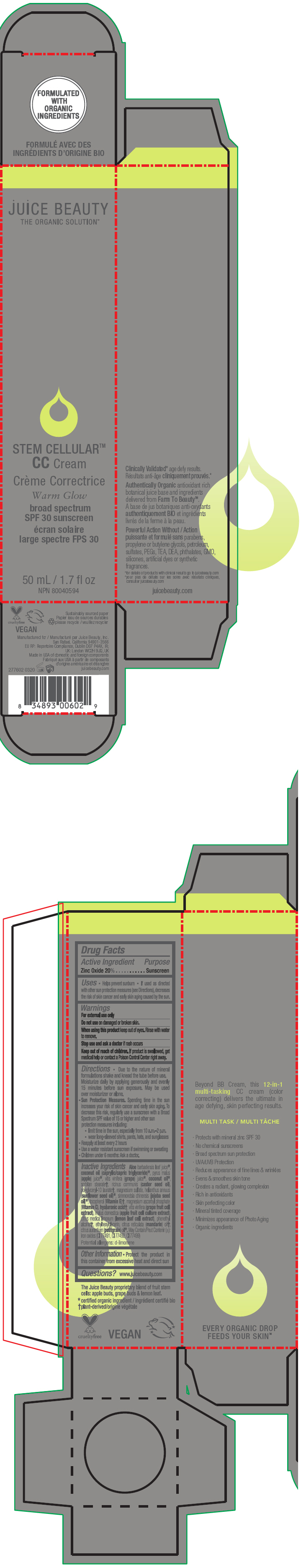
STEM CELLULAR CC CREAM WARM GLOW- zinc oxide lotion
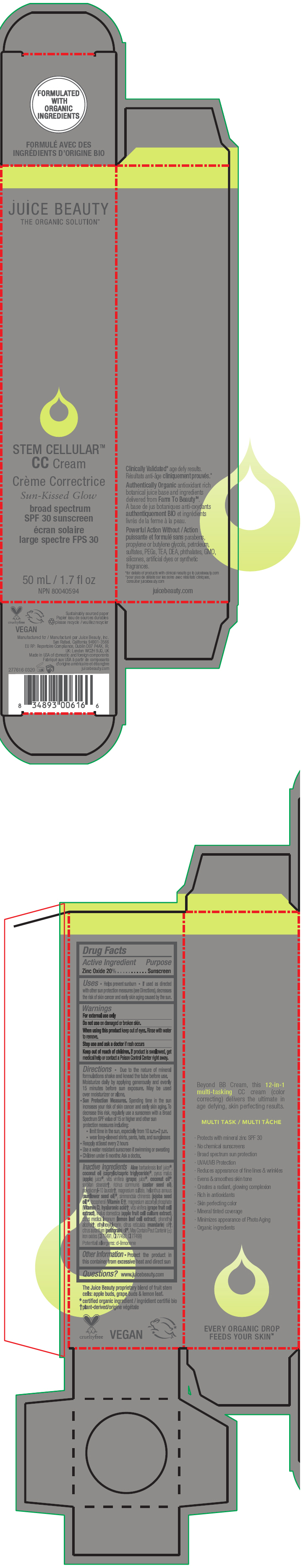
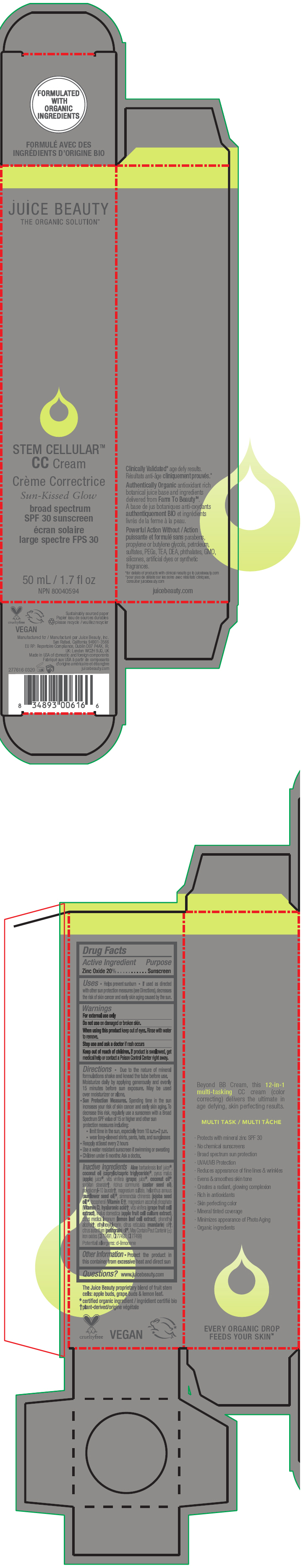
STEM CELLULAR CC CREAM SUN-KISSED GLOW- zinc oxide lotion
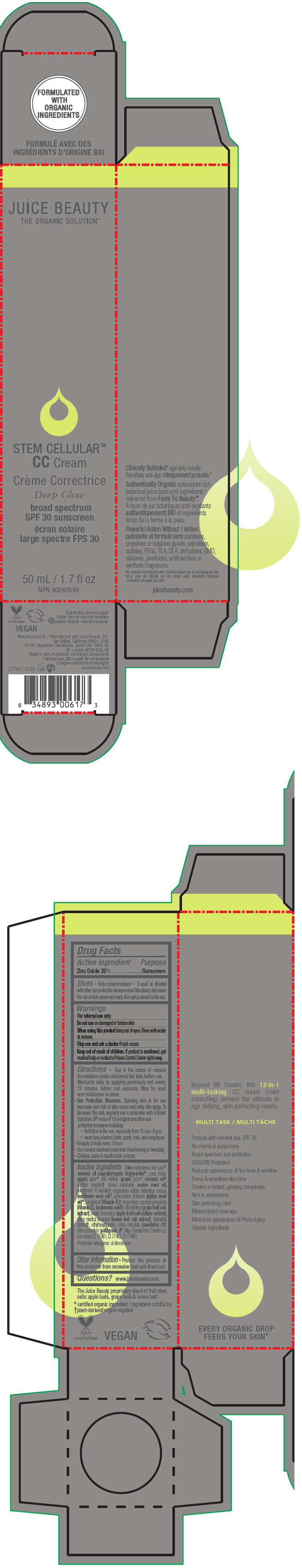
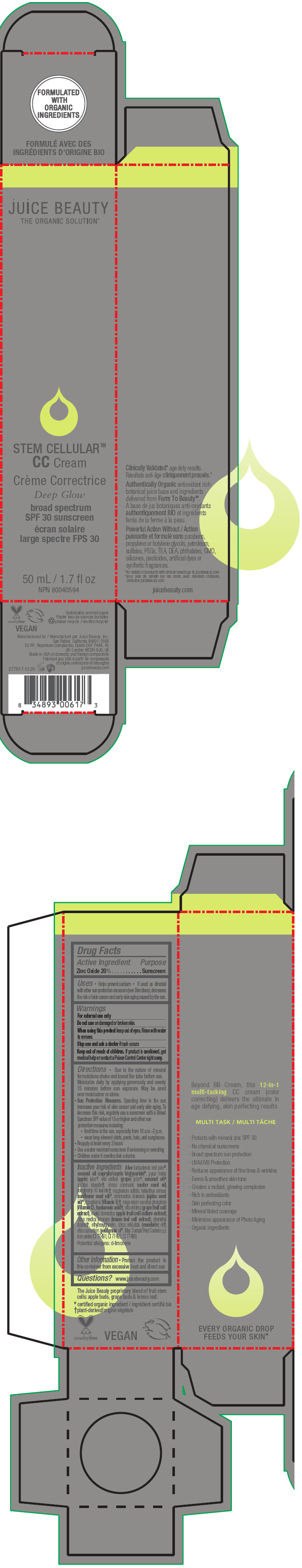
STEM CELLULAR CC CREAM DEEP GLOW- zinc oxide lotion
-
NDC Code(s):
55165-1001-1,
55165-1002-1,
55165-1003-1,
55165-1004-1, view more55165-1005-1, 55165-1006-1, 55165-1007-1
- Packager: Juice Beauty
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Due to the nature of mineral formulations shake and knead the tube before use. Moisturize daily by applying generously and evenly 15 minutes before sun exposure. May be used over moisturizer or alone.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months: Ask a doctor.
-
Inactive Ingredients
Aloe barbadensis leaf juice1, coconut oil (caprylic/capric triglyceride)1, pyrus malus (apple) juice1, vitis vinifera (grape) juice1, coconut oil1, sorbitan stearate2, ricinus communis (castor seed oil), polyglyceryl-10 laurate2, magnesium sulfate, helianthus annuus (sunflower seed oil)1, simmondsia chinensis (jojoba seed oil)1, tocopherol (Vitamin E)2, magnesium ascorbyl phosphate (Vitamin C), hyaluronic acid2, vitis vinifera (grape fruit cell extract), malus domestica (apple fruit cell culture extract), citrus medica limonum (lemon leaf cell extract), phenethyl alcohol2, ethylhexylglycerin, citrus reticulata (mandarin) oil2, citrus aurantium (petitgrain) oil1. May Contain/Peut Contenir (±): iron oxides (CI 77491, CI 77492, CI 77499)
Potential allergens: d-limonene
- Other Information
- Questions?
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - Natural
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - Rosy
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - Beach
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - Desert
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - Warm
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - Sun-Kissed
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - Deep
-
INGREDIENTS AND APPEARANCE
STEM CELLULAR CC CREAM NATURAL GLOW
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55165-1001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength APPLE (UNII: B423VGH5S9) apple juice (UNII: 9871T0PD5P) WINE GRAPE (UNII: 3GOV20705G) ALOE VERA LEAF (UNII: ZY81Z83H0X) coconut oil (UNII: Q9L0O73W7L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) CASTOR OIL (UNII: D5340Y2I9G) polyglyceryl-10 laurate (UNII: MPJ2Q8WI8G) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) magnesium ascorbyl phosphate (UNII: 0R822556M5) hyaluronic acid (UNII: S270N0TRQY) LEMON (UNII: 24RS0A988O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ethylhexylglycerin (UNII: 147D247K3P) phenylethyl alcohol (UNII: ML9LGA7468) mandarin oil (UNII: NJO720F72R) CITRUS AURANTIUM LEAFY TWIG OIL (UNII: 5K6H1IMT3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55165-1001-1 1 in 1 CARTON 12/10/2012 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/10/2012 STEM CELLULAR CC CREAM ROSY GLOW
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55165-1002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength APPLE (UNII: B423VGH5S9) apple juice (UNII: 9871T0PD5P) WINE GRAPE (UNII: 3GOV20705G) ALOE VERA LEAF (UNII: ZY81Z83H0X) coconut oil (UNII: Q9L0O73W7L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) CASTOR OIL (UNII: D5340Y2I9G) polyglyceryl-10 laurate (UNII: MPJ2Q8WI8G) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) magnesium ascorbyl phosphate (UNII: 0R822556M5) hyaluronic acid (UNII: S270N0TRQY) LEMON (UNII: 24RS0A988O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ethylhexylglycerin (UNII: 147D247K3P) phenylethyl alcohol (UNII: ML9LGA7468) mandarin oil (UNII: NJO720F72R) CITRUS AURANTIUM LEAFY TWIG OIL (UNII: 5K6H1IMT3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55165-1002-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 03/10/2020 STEM CELLULAR CC CREAM BEACH GLOW
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55165-1003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength APPLE (UNII: B423VGH5S9) apple juice (UNII: 9871T0PD5P) WINE GRAPE (UNII: 3GOV20705G) ALOE VERA LEAF (UNII: ZY81Z83H0X) coconut oil (UNII: Q9L0O73W7L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) CASTOR OIL (UNII: D5340Y2I9G) polyglyceryl-10 laurate (UNII: MPJ2Q8WI8G) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) magnesium ascorbyl phosphate (UNII: 0R822556M5) hyaluronic acid (UNII: S270N0TRQY) LEMON (UNII: 24RS0A988O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ethylhexylglycerin (UNII: 147D247K3P) phenylethyl alcohol (UNII: ML9LGA7468) mandarin oil (UNII: NJO720F72R) CITRUS AURANTIUM LEAFY TWIG OIL (UNII: 5K6H1IMT3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55165-1003-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 03/10/2020 STEM CELLULAR CC CREAM DESERT GLOW
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55165-1004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength APPLE (UNII: B423VGH5S9) apple juice (UNII: 9871T0PD5P) WINE GRAPE (UNII: 3GOV20705G) ALOE VERA LEAF (UNII: ZY81Z83H0X) coconut oil (UNII: Q9L0O73W7L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) CASTOR OIL (UNII: D5340Y2I9G) polyglyceryl-10 laurate (UNII: MPJ2Q8WI8G) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) magnesium ascorbyl phosphate (UNII: 0R822556M5) hyaluronic acid (UNII: S270N0TRQY) LEMON (UNII: 24RS0A988O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ethylhexylglycerin (UNII: 147D247K3P) phenylethyl alcohol (UNII: ML9LGA7468) mandarin oil (UNII: NJO720F72R) CITRUS AURANTIUM LEAFY TWIG OIL (UNII: 5K6H1IMT3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55165-1004-1 1 in 1 CARTON 12/10/2012 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/10/2012 STEM CELLULAR CC CREAM WARM GLOW
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55165-1005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength APPLE (UNII: B423VGH5S9) apple juice (UNII: 9871T0PD5P) WINE GRAPE (UNII: 3GOV20705G) ALOE VERA LEAF (UNII: ZY81Z83H0X) coconut oil (UNII: Q9L0O73W7L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) CASTOR OIL (UNII: D5340Y2I9G) polyglyceryl-10 laurate (UNII: MPJ2Q8WI8G) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) magnesium ascorbyl phosphate (UNII: 0R822556M5) hyaluronic acid (UNII: S270N0TRQY) LEMON (UNII: 24RS0A988O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ethylhexylglycerin (UNII: 147D247K3P) phenylethyl alcohol (UNII: ML9LGA7468) mandarin oil (UNII: NJO720F72R) CITRUS AURANTIUM LEAFY TWIG OIL (UNII: 5K6H1IMT3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55165-1005-1 1 in 1 CARTON 12/10/2012 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/10/2012 STEM CELLULAR CC CREAM SUN-KISSED GLOW
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55165-1006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength APPLE (UNII: B423VGH5S9) apple juice (UNII: 9871T0PD5P) WINE GRAPE (UNII: 3GOV20705G) ALOE VERA LEAF (UNII: ZY81Z83H0X) coconut oil (UNII: Q9L0O73W7L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) CASTOR OIL (UNII: D5340Y2I9G) polyglyceryl-10 laurate (UNII: MPJ2Q8WI8G) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) magnesium ascorbyl phosphate (UNII: 0R822556M5) hyaluronic acid (UNII: S270N0TRQY) LEMON (UNII: 24RS0A988O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ethylhexylglycerin (UNII: 147D247K3P) phenylethyl alcohol (UNII: ML9LGA7468) mandarin oil (UNII: NJO720F72R) CITRUS AURANTIUM LEAFY TWIG OIL (UNII: 5K6H1IMT3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55165-1006-1 1 in 1 CARTON 12/10/2012 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/10/2012 STEM CELLULAR CC CREAM DEEP GLOW
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55165-1007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength APPLE (UNII: B423VGH5S9) apple juice (UNII: 9871T0PD5P) WINE GRAPE (UNII: 3GOV20705G) ALOE VERA LEAF (UNII: ZY81Z83H0X) coconut oil (UNII: Q9L0O73W7L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) CASTOR OIL (UNII: D5340Y2I9G) polyglyceryl-10 laurate (UNII: MPJ2Q8WI8G) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) magnesium ascorbyl phosphate (UNII: 0R822556M5) hyaluronic acid (UNII: S270N0TRQY) LEMON (UNII: 24RS0A988O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ethylhexylglycerin (UNII: 147D247K3P) phenylethyl alcohol (UNII: ML9LGA7468) mandarin oil (UNII: NJO720F72R) CITRUS AURANTIUM LEAFY TWIG OIL (UNII: 5K6H1IMT3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55165-1007-1 1 in 1 CARTON 12/10/2012 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/10/2012 Labeler - Juice Beauty (263151582)