Label: NOCDURNA- desmopressin acetate tablet
- NDC Code(s): 54436-325-10, 54436-325-30, 54436-350-10, 54436-350-30
- Packager: Antares Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 19, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NOCDURNA ® safely and effectively. See full prescribing information for NOCDURNA.
NOCDURNA (desmopressin acetate) sublingual tablets
Initial U.S. Approval: 1978WARNING: HYPONATREMIA
See full prescribing information for complete boxed warning.
- NOCDURNA can cause hyponatremia, which may be life-threatening if severe (5.1)
- NOCDURNA is contraindicated in patients at increased risk of severe hyponatremia, such as patients with excessive fluid intake, illnesses that can cause fluid or electrolyte imbalances, and in those using loop diuretics or systemic or inhaled glucocorticoids. (4, 5.1)
- Ensure serum sodium concentration is normal before starting or resuming NOCDURNA. Measure serum sodium within 1 week and approximately 1 month after initiating therapy and periodically during treatment. More frequently monitor serum sodium in patients 65 years of age and older and in patients at increased risk of hyponatremia (2.2,5.1)
- If hyponatremia occurs, NOCDURNA may need to be temporarily or permanently discontinued (5.1)
INDICATIONS AND USAGE
NOCDURNA is a vasopressin analog indicated for the treatment of nocturia due to nocturnal polyuria in adults who awaken at least 2 times per night to void. (1)
DOSAGE AND ADMINISTRATION
Dosing Information (2.1):
- Women: 27.7 mcg daily, one hour before bedtime, administered sublingually without water
- Men: 55.3 mcg daily, one hour before bedtime, administered sublingually without water
DOSAGE FORMS AND STRENGTHS
Sublingual Tablets: 27.7 mcg of desmopressin acetate (equivalent to 25 mcg of desmopressin) and 55.3 mcg of desmopressin acetate (equivalent to 50 mcg of desmopressin) (3)
CONTRAINDICATIONS
- Hyponatremia or a history of hyponatremia (4)
- Polydipsia (4)
- Concomitant use with loop diuretics or systemic or inhaled glucocorticoids (4)
- Estimated glomerular filtration rate below 50 mL/min/1.73 m2 (4)
- Syndrome of inappropriate antidiuretic hormone secretion (SIADH) (4)
- During illnesses that can cause fluid or electrolyte imbalance (4)
- Heart failure (4)
- Uncontrolled hypertension (4)
WARNINGS AND PRECAUTIONS
- Limit fluid intake to a minimum from 1 hour before until 8 hours after administration. Treatment without concomitant reduction of fluid intake may lead to fluid retention and hyponatremia (5.1)
- Fluid retention: Not recommended in patients at risk of increased intracranial pressure or history of urinary retention. (5.2)
ADVERSE REACTIONS
Common adverse reactions (> 2% incidence) included dry mouth, hyponatremia or blood sodium decreased, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ferring at 1-888-337-7464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Monitor serum sodium more frequently when NOCDURNA is concomitantly used with drugs that may increase the risk of hyponatremia (e.g., tricyclic antidepressants, selective serotonin re-uptake inhibitors, chlorpromazine, opiate analgesics, thiazide diuretics, NSAIDs, lamotrigine, chlorpropamide and, carbamazepine). (7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: HYPONATREMIA
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Recommendations
2.2 Sodium Monitoring
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hyponatremia
5.2 Fluid Retention
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
7 DRUG INTERACTIONS
7.1 Drugs That May Increase the Risk of Hyponatremia
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: HYPONATREMIA
NOCDURNA can cause hyponatremia. Severe hyponatremia can be life-threatening, leading to seizures, coma, respiratory arrest, or death [see Warnings and Precautions (5.1)].
NOCDURNA is contraindicated in patients at increased risk of severe hyponatremia, such as patients with excessive fluid intake, illnesses that can cause fluid or electrolyte imbalances, and in those using loop diuretics or systemic or inhaled glucocorticoids [see Contraindications (4) and Warnings and Precautions (5.1)].
Ensure the serum sodium concentration is normal before starting or resuming NOCDURNA. Measure serum sodium within 7 days and approximately 1 month after initiating therapy, and periodically during treatment. More frequently monitor serum sodium in patients 65 years of age and older and in patients at increased risk of hyponatremia. [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
If hyponatremia occurs, NOCDURNA may need to be temporarily or permanently discontinued [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
NOCDURNA is indicated for the treatment of nocturia due to nocturnal polyuria in adults who awaken at least 2 times per night to void.
In the NOCDURNA clinical trials nocturnal polyuria was defined as night-time urine production exceeding one-third of the 24-hour urine production.
Before starting NOCDURNA:
- Evaluate the patient for possible causes for the nocturia, including excessive fluid intake prior to bedtime, and address other treatable causes of nocturia.
- Confirm the diagnosis of nocturnal polyuria with a 24-hour urine collection, if one has not been obtained previously.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Recommendations
Before starting or resuming NOCDURNA assess the sodium concentration and only start or resume NOCDURNA in patients with a normal serum sodium concentration [see Warnings and Precautions (5.1), Contraindications (4)].
The recommended NOCDURNA dosage in:
- Women is 27.7 mcg once daily, one hour before bedtime, administered sublingually without water.
- Men is 55.3 mcg once daily, one hour before bedtime, administered sublingually without water.
Keep the tablet under the tongue until it has fully dissolved.
The recommended dose for women is lower than for men because women are more sensitive to the effects of NOCDURNA and had a higher risk of hyponatremia with the 55.3 mcg dose in clinical trials.
Instruct patients to empty their bladder immediately before bedtime. Limit fluid intake to a minimum from 1 hour before until 8 hours after administration [see Warnings and Precautions (5.1) and Patient Counseling Information (17)].
2.2 Sodium Monitoring
Ensure serum sodium concentration is normal prior to initiating or resuming NOCDURNA. NOCDURNA is contraindicated in patients with hyponatremia or a history of hyponatremia [see Contraindications (4)].
Check the serum sodium concentration within the first week and again at one month after initiating or resuming therapy.
Periodically monitor serum sodium during NOCDURNA therapy, as clinically appropriate. More frequent serum sodium monitoring is recommended for patients 65 years and older and for those at risk of hyponatremia.
If the patient develops hyponatremia, NOCDURNA may need to be temporarily or permanently discontinued, and treatment for the hyponatremia instituted, depending on the clinical circumstances, including the duration and severity of the hyponatremia [see Warnings and Precautions (5.1)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
NOCDURNA is contraindicated in patients with the following conditions due to an increased risk of hyponatremia:
- Hyponatremia or a history of hyponatremia [see Warnings and Precautions (5.1)].
- Polydipsia
- Concomitant use with loop diuretics [see Warnings and Precautions (5.1)]
- Concomitant use with systemic or inhaled glucocorticoids [see Warnings and Precautions (5.2), Drug Interactions (7.1)]
- Renal impairment with estimated glomerular filtration rate (eGFR) below 50 mL/min/1.73 m2 [see Use in Specific Population (8.6) and Clinical Pharmacology (12.3)].
- Known or suspected syndrome of inappropriate antidiuretic hormone (SIADH) secretion.
- During illnesses that can cause fluid or electrolyte imbalance, such as gastroenteritis, salt-wasting nephropathies, or systemic infection
NOCDURNA is contraindicated in patients with the following conditions because fluid retention increases the risk of worsening the underlying condition:
- Heart failure [See Warnings and Precautions (5.2)]
- Uncontrolled hypertension
-
5 WARNINGS AND PRECAUTIONS
5.1 Hyponatremia
NOCDURNA can cause hyponatremia [see Boxed Warning and Adverse Reactions (6.1)]. Severe hyponatremia can be life-threatening if it is not promptly diagnosed and treated, leading to seizures, coma, respiratory arrest, or death.
NOCDURNA is contraindicated in patients at increased risk of severe hyponatremia, such as those with excessive fluid intake, those who have illnesses that can cause fluid or electrolyte imbalances, and in those using loop diuretics or systemic or inhaled glucocorticoids [see Contraindications (4), and Drug Interactions (7.1)].
Prior to starting or resuming NOCDURNA, ensure that the serum sodium concentration is normal.
Limit fluid intake to a minimum from 1 hour before administration until 8 hours after administration. Use of NOCDURNA without concomitant reduction of fluid intake may lead to fluid retention and hyponatremia. Advise patients to avoid drinks containing caffeine or alcohol before bedtime. Monitor the serum sodium concentration within 1 week and approximately 1 month of initiating NOCDURNA, and periodically thereafter. The frequency of serum sodium monitoring should be based on the patient's risk for hyponatremia. The incidence of hyponatremia was higher in patients 65 years of age or older compared to younger patients. More frequent monitoring is recommended for patients 65 years of age or older or those on concomitant medications that can increase the risk of hyponatremia, such as tricyclic antidepressants, selective serotonin reuptake inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), chlorpromazine, opiate analgesics, carbamazepine, lamotrigine, thiazide diuretics and chlorpropamide [see Drug Interactions (7.1)].
If hyponatremia occurs, NOCDURNA may need to be temporarily or permanently discontinued and treatment for the hyponatremia instituted, depending on the clinical circumstances, including the duration and severity of the hyponatremia.
Women are more sensitive to the effects of NOCDURNA compared to men [see Clinical Pharmacology (12.2)]. The recommended dose for women is lower than for men because women had a higher risk of hyponatremia with the 55.3 mcg dose in clinical trials.
5.2 Fluid Retention
NOCDURNA can cause fluid retention, which can worsen underlying conditions that are susceptible to volume status. Therefore, NOCDURNA is contraindicated in patients with heart failure or uncontrolled hypertension [see Contraindications (4)]. In addition, NOCDURNA is not recommended in patients at risk for increased intracranial pressure or those with a history of urinary retention.
-
6 ADVERSE REACTIONS
The following adverse reaction is described elsewhere in the labeling:
- Hyponatremia [see Boxed Warning and Warnings and Precautions (5.1)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety database includes three double-blind, placebo-controlled, multicenter, randomized trials of NOCDURNA and one open-label extension trial. Study 1 (CS40) (NCT01262456) enrolled only women, Study 2 (CS41) (NCT01223937) enrolled only men, Study 3 (CS29) (NCT00477490) enrolled men and women and Study 4 (CS31) (NCT00615836) was an extension of Study 3 for up to 3 years [see Clinical Studies (14)].
At baseline, 196 women treated with NOCDURNA 27.7 mcg/day, 173 women given placebo, 195 men treated with NOCDURNA 55.3 mcg/day, and 213 men given placebo had nocturia due to nocturnal polyuria, with at least 2 nocturnal voids per night. The mean age of women treated with NOCDURNA 27.7 mcg was 59 years and 42% of women were aged 65 and older. The mean age of men treated with NOCDURNA 55.3 mcg was 62 years and 50% of men were aged 65 and older. Caucasians comprised 81%, Blacks 17%, and Asians 1% of the nocturia due to nocturnal polyuria population, and 12% were Hispanic.
Concomitant use of anti-muscarinic medications, alpha-blockers, and alpha-reductase inhibitors was permitted for patients on a stable dose prior to study entry. Serious adverse reactions included 2 reports of hyponatremia in men treated with NOCDURNA 55.3 mcg. These 2 reports occurred in a trial in which all cases of serum sodium ≤125 mmol/L were reported as serious adverse reactions.
Adverse Reactions Leading to Discontinuation
Among women with nocturia due to nocturnal polyuria, the discontinuation rate due to adverse reactions was 3% for those treated with NOCDURNA 27.7 mcg and 2% for those in the placebo group. Among men with nocturia due to nocturnal polyuria, the discontinuation rate due to adverse reactions was 4% for those treated with NOCDURNA 55.3 mcg and 3% in the placebo group.
Table 1 displays the most common adverse reactions leading to discontinuation in patients with nocturia due to nocturnal polyuria.
Table 1: Most Common Adverse Reactions (≥2 Incidences) Leading to Discontinuation in Patients With Nocturia Due to Nocturnal Polyuria (Studies 1, 2 and 3)* Women Men Adverse Reactions Placebo
(N=173)NOCDURNA
27.7 mcg
(N=196)Placebo
(N=213)NOCDURNA
55.3 mcg
(N=195)- *
- Includes adverse reactions occurring during up to 3 months of treatment in patients who continued from Study 3 into Study 4
Hyponatremia or blood sodium decreased 1 (<1%) 1 (<1%) 0 4 (2.1%) Most Common Adverse Reactions
Table 2 displays the most common adverse reactions in patients with nocturia due to nocturnal polyuria in Studies 1, 2, and 3. The most common adverse reactions reported with both the 27.7 mcg/day and 55.3 mcg/day dosages included dry mouth, hyponatremia or blood sodium decreased, and dizziness.
The high incidence of dry mouth may have been affected by specific query about dry mouth in Study 3 (CS29). In Studies 1 and 2 where the adverse reaction was spontaneously reported, the incidence was ≤4%.
Table 2: Common Adverse Reactions (Reported by >2% of NOCDURNA-Treated Patients and at a Higher Incidence With Either Dose Than With Placebo) in Patients With Nocturia Due to Nocturnal Polyuria (Studies 1, 2 and 3)* Women Men Adverse Reactions Placebo
(N=173)NOCDURNA
27.7 mcg
(N=196)Placebo
(N=213)NOCDURNA
55.3 mcg
(N=195)- *
- Includes adverse reactions occurring during up to 3 months of treatment in patients who continued from Study 3 into Study 4
Dry mouth 19 (11%) 23 (12%) 27 (13%) 27 (14%) Hyponatremia or blood sodium decreased 3 (2%) 6 (3%) 1 (<1%) 8 (4%) Headache 5 (3%) 4 (2%) 3 (1%) 7 (4%) Dizziness 0 3 (2%) 1 (<1%) 5 (3%) Hyponatremia
Serum sodium was measured during screening, at baseline, and on all study visits during treatment including day 4, week 1, week 2 (males only), week 4 and then every month of the studies. Tables 3 and Table 4 show the incidence of serum sodium concentrations below the normal range based on the pooled analysis of three phase 3 studies.
Table 3: Incidence of Hyponatremia by Sex in Patients with Nocturia due to Nocturnal Polyuria (Studies 1, 2, and 3)* Women Men Serum Sodium
(mmol/L)Placebo
(N=171)NOCDURNA 27.7 mcg/day
(N=191)Placebo
(N=207)NOCDURNA
55.3 mcg/day
(N=192)Some subjects received different doses over the course of Study 3 and are in more than one dose-group. n is number observed post baseline - *
- Includes adverse reactions occurring during up to 3 months of treatment in patients who continued from Study 3 into Study 4
130-134 7 (4%) 13 (7%) 6 (3%) 33 (17%) 126-129 0 (0%) 7 (4%) 0 (0%) 1 (<1%) ≤125 1 (<1%) 0 (0%) 0 (0%) 3 (2%) Table 4: Incidence of Hyponatremia by Sex and Age in Patients with Nocturia due to Nocturnal Polyuria (Studies 1, 2 and 3)* Serum Sodium
(mmol/L)Women
<65 yearsWomen
≥ 65 yearsMen
< 65 yearsMen
≥ 65 yearsPlacebo
(N=95)NOCDURNA
27.7 mcg/day
(N=113)Placebo
(N=76)NOCDURNA
27.7 mcg/day
(N=78)Placebo
(N=95)NOCDURNA
55.3 mcg/day
(N=98)Placebo
(n=112)NOCDURNA
55.3 mcg/day
(N=94)Some subjects received different doses over the course of study 3 and are in more than one dose-group. n is number observed post baseline - *
- Includes adverse reactions occurring during up to 3 months of treatment in patients who continued from Study 3 into Study 4
130-134 2 (2%) 4 (4%) 5 (7%) 9 (12%) 5 (5%) 11 (11%) 1 (<1%) 22 (23%) 126-129 0 (0%) 2 (2%) 0 (0%) 5 (6%) 0 (0%) 0 (0%) 0 (0%) 1 (1%) ≤125 0 (0%) 0 (0%) 1 (1%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 3 (3%) -
7 DRUG INTERACTIONS
7.1 Drugs That May Increase the Risk of Hyponatremia
Concomitant use of NOCDURNA and loop diuretics or systemic or inhaled glucocorticoids is contraindicated because of the risk of severe hyponatremia [see Boxed Warning, Contraindications (4), and Warnings and Precautions (5.1)]. NOCDURNA can be started or resumed three days or five half-lives after the glucocorticoid is discontinued, whichever is longer.
Drugs such as tricyclic antidepressants, selective serotonin reuptake inhibitors, chlorpromazine, opiate analgesics, thiazide diuretics, carbamazepine, lamotrigine, sulfonylureas, particularly chlorpropamide, and NSAIDs may increase the risk of hyponatremia. Monitor serum sodium more frequently in patients taking NOCDURNA concomitantly with these drugs and when doses of these drugs are increased [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
NOCDURNA is not recommended for the treatment of nocturia in pregnant women. Nocturia is usually related to normal, physiologic changes during pregnancy that do not require treatment with NOCDURNA.
There are no data with NOCDURNA use in pregnant women to inform any drug-associated risks. No adverse developmental outcomes were observed in animal reproductive and developmental studies following administration of desmopressin acetate during organogenesis to pregnant rats and rabbits, at exposures 92- and 8- times, respectively, the maximum recommended dose in women, based on body surface area (mg/m2) (see Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Desmopressin acetate, administered during organogenesis did not cause fetal harm in teratology studies in rats at intravenous doses up to 238 mcg/kg/day or in rabbits at subcutaneous doses up to 10 mcg/kg/day, representing 92- and 8-times, respectively, the maximum recommended dose in women of 27.7 mcg, based on body surface area (mg/m2).
8.2 Lactation
Risk Summary
Desmopressin is present in small amounts in human milk (see Data).
There is no information on the effects of desmopressin on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's need for NOCDURNA and any potential adverse effects on the breastfed child from NOCDURNA or from the underlying maternal condition.
Data
Human Data
The breast milk of lactating women was collected over 8 hours following desmopressin (300 mcg) administration using nasal spray. Based on the measured concentrations of desmopressin, the amounts of desmopressin that may be transferred to a breastfed infant correspond to 0.0001-0.0005% of the dose administered.
8.4 Pediatric Use
The safety and effectiveness of NOCDURNA have not been established in pediatric patients.
8.5 Geriatric Use
A total of 562 subjects 65 years or older were enrolled in the clinical trials, about 48% of the study population.
Clinical studies of desmopressin have shown an increased risk of hyponatremia in patients 65 years of age or older compared to those younger than 65 years of age [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
8.6 Renal Impairment
No dose adjustment of NOCDURNA is required for patients with an eGFR at or above 50 mL/min/1.73 m2. NOCDURNA is contraindicated in patients with an eGFR below 50 mL/min/1.73 m2 [see Contraindications (4)].
-
10 OVERDOSAGE
Overdosage of desmopressin leads to an increased risk of prolonged fluid retention and hyponatremia. Signs of overdosage may include nausea, headache, drowsiness, confusion, and rapid weight gain due to fluid retention [See Warnings and Precautions (5.1)].
In case of overdosage, NOCDURNA must be discontinued, serum sodium assessed, and hyponatremia treated appropriately.
-
11 DESCRIPTION
NOCDURNA is a sublingual tablet containing desmopressin acetate, a synthetic analog of the endogenous pituitary hormone, 8-arginine vasopressin (ADH), an antidiuretic hormone. It is chemically defined as follows:
Molecular weight of 1183.34 with the following empirical formula:
C46H64N14O12S2∙C2H4O2∙3H2O
1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate.
NOCDURNA (desmopressin acetate) sublingual tablets are available in two strengths. Each sublingual tablet contains 27.7 mcg or 55.3 mcg of desmopressin acetate, equivalent to 25 mcg or 50 mcg of desmopressin as free base, respectively. The inactive ingredients are gelatin, NF (fish source), mannitol, and anhydrous citric acid.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The antidiuretic effects of desmopressin are mediated by stimulation of vasopressin 2 (V2) receptors, thereby increasing water re-absorption in the kidneys, and reducing urine production.
12.2 Pharmacodynamics
In a pharmacodynamic study following sublingual administration of 60 mcg desmopressin (1.2 and 2.4 times the maximum recommended dose in men and women, respectively) with suppression of the endogenous vasopressin release by continuous intake of water, the mean time to onset of antidiuretic action was observed within 30 minutes and lasted 6 hours after dosing.
In a study in patients with nocturia due to nocturnal polyuria, the weight-corrected NOCDURNA dose that induced 50% maximum achievable drug effect on nocturnal urine volume (ED50) differed significantly between women and men. The ED50 value for men was 2.7-fold (95% CI 1.3-8.1) higher than the value for women, corresponding to higher desmopressin sensitivity among women [see Dosage and Administration (2.1)].
Dose, sex, age and renal impairment affect the risk of developing hyponatremia [see Warnings and Precautions (5.1)].
12.3 Pharmacokinetics
The pharmacokinetics of desmopressin following sublingual administration of NOCDURNA has not been characterized. The pharmacokinetic information provided below is from studies following sublingual administration of higher doses or intravenous injection of desmopressin.
Absorption
The overall mean absolute bioavailability of desmopressin administered sublingually (at doses of 200, 400 and 800 mcg, which represents 4, 8, and 16 times the maximum recommend dosage in men) was 0.25% (95% CI 0.21–0.31%).
Distribution
The volume of distribution of desmopressin after intravenous administration of 2 mcg is 26.5 L.
Elimination
The geometric mean terminal half-life is 2.8 hours.
Drug Interaction Studies
In vitro studies in human liver microsome preparations have shown that desmopressin does not inhibit the human CYP450 system. No in vivo interaction studies have been performed with NOCDURNA.
Patients with Renal Impairment
A pharmacokinetic study was conducted in subjects with normal renal function and patients with mild, moderate, and severe renal impairment (n=24, 6 subjects in each group) who received a single 2 mcg dose of desmopressin intravenous injection.
The geometric mean terminal half-life was 2.8 hours in subjects with normal renal function, and 4, 6.6, and 8.7 hours in patients with mild, moderate, and severe renal impairment, respectively. In patients with mild, moderate and severe renal impairment, mean desmopressin area under the plasma drug concentration time curve (AUC) was 1.5-fold, 2.4-fold and 3.7-fold higher, respectively, compared to that of subjects with normal renal function [see Contraindications (4), Use in Specific Populations (8.6)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with desmopressin have not been performed to evaluate carcinogenic potential.
Desmopressin was not mutagenic in bacterial mutagenicity (Ames) and mouse lymphoma assays.
Animal studies with desmopressin showed no impairment of fertility in male and female rats at doses up to 200 mcg/kg/day.
-
14 CLINICAL STUDIES
The efficacy of NOCDURNA in the treatment of adults with nocturia due to nocturnal polyuria was established in two 3-month randomized, double-blind, placebo-controlled, multicenter trials in adults over 18 years of age. Study 1 enrolled only women and Study 2 enrolled only men. At baseline, patients were required to document at least two nocturnal voids per night in a consecutive 3-day diary collected during screening. Randomization for studies 1 and 2 was stratified by age group (<65 vs. ≥65 years). Subjects with severe daytime voiding dysfunction and other possible causes of nocturia (e.g., uncontrolled diabetes mellitus, obstructive sleep apnea) were excluded.
In Studies 1 and 2, the mean age was approximately 60 years and the ethnic/racial distribution was approximately 80% Caucasian, 20% African American, and 1% Asian.
In Study 1, a total of 237 women with nocturia due to nocturnal polyuria were randomized to receive either sublingual NOCDURNA 27.7 mcg (n=121) or placebo (n=116) every night approximately 1 hour prior to bedtime for 3 months. In Study 2, a total of 230 men with nocturia due to nocturnal polyuria were randomized to receive sublingual NOCDURNA 55.3 mcg (n=102) or placebo (n=128) every night approximately 1 hour prior to bedtime for 3 months. Nocturnal polyuria was defined as nighttime urine production exceeding one-third of the 24-hour urine production confirmed with a 24-hour urine frequency/volume chart.
The co-primary efficacy endpoints in each trial were 1) the change in number of nocturia episodes per night from baseline during the 3-month treatment period, and 2) 33% responder status during three months of treatment. A 33% responder was defined as a subject with a decrease of at least 33% in the mean number of nocturnal voids compared to baseline.
Many conditions can cause nocturia. The efficacy and safety of NOCDURNA have not been established for the treatment of all causes of nocturia. NOCDURNA is indicated only for patients who have nocturia due to nocturnal polyuria.
The results for the co-primary efficacy endpoints among patients with nocturia due to nocturnal polyuria are shown in Table 5.
Table 5. Primary Efficacy Results in Subjects with Nocturia due to Nocturnal Polyuria in Studies 1 and 2 (mITT population) Women (Study 1) Men (Study 2) Placebo NOCDURNA
27.7 mcg once dailyPlacebo NOCDURNA
55.3 mcg once dailyN=114 N=118 N=128 N=102 mITT: modified Intent-to-Treat (including all randomized patients who received at least one dose of study drug) CI = confidence interval - *
- Repeated measures ANCOVA of change from baseline at Week 1, Month 1, Month 2 and Month 3, adjusted for age stratification factor (<65, ≥65 years), visit, and baseline nocturnal voids
- †
- GEE Method for 33% responder status at Week 1, Month 1, Month 2 and Month 3, adjusted for age stratification factor (<65, ≥65 years), visit, and baseline nocturnal voids
Mean number of nocturnal voids Baseline (mean) 2.9 2.9 3.0 3.0 Change from baseline* -1.2 -1.5 -0.9 -1.3 Difference from placebo* -0.3 -0.4 95% CI* (-0.5, -0.1) (-0.6, -0.2) 33% responder status Probability† 0.62 0.78 0.50 0.67 Odds Ratio† 2.15 2.02 95% CI† (1.36, 3.41) (1.30, 3.14) To help interpret the clinical meaningfulness of the efficacy findings, results of the additional analyses for the percentage of nights during the 3-month treatment period with no nocturia and the percentage of nights during the 3-month treatment period with at most one nocturia episode, are displayed in Table 6.
Table 6 Summary of Additional Analysis Results in Subjects with Nocturia due to Nocturnal Polyuria in Studies 1 and 2 (mITT population) Women (Study 1) Men (Study 2) Placebo NOCDURNA
27.7 mcg once dailyPlacebo NOCDURNA
55.3 mcg once dailyN=114 N=118 N=128 N=102 mITT: modified Intent-to-Treat (including all randomized patients who received at least one dose of study drug) CI: confidence interval - *
- ANCOVA model adjusted for treatment, age stratification factor (<65, ≥65 years), and baseline nocturnal voids
Percentage of nights with at most one nocturnal void Baseline (mean) 0% 1% 1% 0% Percentage* 45% 58% 32% 44% Difference from placebo* 13% 11% 95% CI* (4%, 21%) (3%, 20%) Percentage of nights with no nocturnal voids Baseline (mean) 0% 0% 0% 0% Percentage* 15% 19% 7% 15% Difference from placebo* 4% 9% 95% CI* (-3%, 11%) (4%, 14%) -
16 HOW SUPPLIED/STORAGE AND HANDLING
NOCDURNA (desmopressin acetate) sublingual tablets are available as:
27.7 mcg of desmopressin acetate (equivalent to 25 mcg of desmopressin): White, round, sublingual tablet with "25" on one side.
NDC 54436-325-30 Carton of 30 sublingual tablets (3 blister packs with 10 tablets each) 55.3 mcg of desmopressin acetate (equivalent to 50 mcg of desmopressin): White, round, sublingual tablet with "50" on one side.
NDC 54436-350-30 Carton of 30 sublingual tablets (3 blister packs with 10 tablets each) -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Fluid Restriction, Hyponatremia, Sodium Monitoring and Acute Illnesses
- Instruct patients to place one tablet under the tongue one hour before bedtime and to empty their bladder immediately prior to bedtime. The tablet should remain under the tongue until it dissolves. [see Dosage and Administration (2.1)].
- Advise patients to limit fluid intake to a minimum starting one hour prior to NOCDURNA administration and for eight hours following NOCDURNA administration. Advise patients to avoid caffeine and alcohol before bedtime [see Warnings and Precautions (5.1)].
- Inform patients that NOCDURNA may cause severe hyponatremia, which may be life-threatening, if it is not promptly diagnosed and treated. Inform them about the signs and symptoms associated with hyponatremia, to undergo recommended serum sodium measurements, and to inform their health care provider about new medications [see Warnings and Precautions (5.1)].
- Inform patients that NOCDURNA should be stopped during acute intercurrent illnesses that cause fluid or electrolyte imbalance [see Warnings and Precautions (5.2)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
NOCDURNA® (knock-DUHR-nah)
(desmopressin acetate) sublingual tablets
What is the most important information I should know about NOCDURNA?
NOCDURNA may cause serious side effects, including:
- Low levels of salt (sodium) in your blood (hyponatremia). Low levels of salt in your blood is a serious side effect of NOCDURNA that may be life threatening, causing seizures, coma, trouble breathing or death, if not treated early.
Stop taking NOCDURNA and call your healthcare provider if you have any of the following symptoms of low salt levels in your blood:
- headache
- feeling restless
- drowsiness
- muscle cramps
- nausea or vomiting
- tiredness (fatigue)
- dizziness
- change in your mental condition such as hallucinations, confusion, decreased awareness or alertness
- You should not take NOCDURNA if you are at risk for very low salt levels in your blood, for example, if you drink a lot of fluid, have illnesses that can cause you to have fluid or body salt (electrolyte) imbalances, if you take a certain type of "water-pill" called a loop diuretic or take glucocorticoids including inhaled steroids.
- Tell your healthcare provider if you have a fever, infection, or diarrhea while taking NOCDURNA as these can cause you to have fluid or body salt (electrolyte) imbalance. Your healthcare provider may tell you not to take NOCDURNA while you have these symptoms.
-
Your healthcare provider should check your blood salt levels:
- before you start or restart taking NOCDURNA.
- within the first week after you start NOCDURNA.
- 1 month after you start NOCDURNA.
- every so often as told to you by your healthcare provider, with testing more often if you are already at risk for low salt levels, for example if you are 65 years or older or take certain medicines that increase your risk of low salt levels.
See "What are the possible side effects of NOCDURNA?" for more information about side effects.
What is NOCDURNA?
NOCDURNA is a prescription medicine used in adults who wake up at least 2 times during the night to urinate due to a condition called nocturnal polyuria. Nocturnal polyuria is a condition where your body makes too much urine at night.
There are other conditions that could cause you to wake up during the night to urinate. NOCDURNA is only approved for the treatment of nocturnal polyuria.
Your doctor should have you measure your urine and the times that you urinate for 24 hours to determine if you have nocturnal polyuria, if you have not already done this.
It is not known if NOCDURNA is safe and effective in children.
Do not take NOCDURNA if you:
- have or have had low salt levels in your blood.
- are thirsty much of the time and drink large amounts of fluids (polydipsia).
- are taking a type of water pill called a loop-diuretic.
- are taking a glucocorticoid (steroid) medicine, including an inhaled glucocorticoid (steroid) medicine.
- have moderate or severe kidney disease.
- have or may have a condition called syndrome of inappropriate antidiuretic hormone (SIADH) secretion.
- have an illness that can cause you to have low levels of fluid or electrolytes in your blood such as vomiting, diarrhea, an infection, or a kidney problem that causes you to have low levels of salt.
- have a heart condition called heart failure.
- have high blood pressure that is not controlled.
- are allergic to any ingredient in NOCDURNA tablets (see a complete list of ingredients at the end of this Medication Guide).
Talk to your healthcare provider before you take NOCDURNA if you have any of these conditions or take any of these medicines.
Before taking NOCDURNA, tell your healthcare provider about all of your medical conditions, including if you:
- are at risk for low salt levels in your blood.
- currently have vomiting, diarrhea, fever or an infection.
- have any heart or kidney problems.
- have high blood pressure.
- have increased pressure in your brain (increased intracranial pressure).
- have a history of not being able to empty your bladder all the way (urinary retention).
- are pregnant or planning to become pregnant. It is not known if NOCDURNA can harm your unborn baby. NOCDURNA is not recommended to treat normal symptoms of pregnancy that cause pregnant women to urinate often at night.
- are breastfeeding or plan to breastfeed. Desmopressin, an ingredient in NOCDURNA, passes into breastmilk. Talk to your healthcare provider about the best way to feed your baby if you take NOCDURNA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using NOCDURNA with certain medicines may cause serious side effects. Do not start taking any new medicines until you talk to your healthcare provider.
Especially tell your healthcare provider if you take a:
- water pill (diuretic).
- glucocorticoid (steroid) medicine, including an inhaled glucocorticoid (steroid) medicine.
- your doctor should stop your treatment with NOCDURNA for a period of time while you are taking and after you stop taking an oral or inhaled glucocorticoid (steroid) medicine
- medicine used to treat depression called a tricyclic antidepressant or selective serotonin reuptake inhibitor (SSRI).
- medicine used to treat mood disorders, such as schizophrenia or bipolar disorder called chlorpromazine.
- medicine used to treat seizures, nerve pain, or bipolar disorder called carbamazepine.
- non-steroidal anti-inflammatory medicine (NSAID).
Ask your healthcare provider or pharmacist if you are not sure if your medicine is one that is listed above.
Tell your healthcare provider if you have fever, infection, or diarrhea while taking NOCDURNA as these can cause you to have fluid or body salt (electrolyte) imbalance. Your healthcare provider may tell you not to take NOCDURNA while you have these symptoms.
How should I take NOCDURNA?
- You should take NOCDURNA 1 time each day, 1 hour before bedtime without water.
- When you are ready to take your dose of NOCDURNA:
- Place the tablet under your tongue 1 hour before bedtime. Leave the tablet under your tongue until it dissolves.
- Empty your bladder just before bedtime.
- While taking NOCDURNA, you should limit the amount of water or liquids you drink from 1 hour before taking NOCDURNA and until 8 hours after. You may have serious side effects if you drink too much liquid.
- You should avoid drinks containing caffeine and alcohol before bedtime as this can cause your body to make more urine.
- Do not take more NOCDURNA than prescribed for you. If you take too much NOCDURNA, call your healthcare provider right away or get emergency treatment.
What are the possible side effects of NOCDURNA?
NOCDURNA may cause serious side effects, including:
- See "What is the most important information I should know about NOCDURNA?"
The most common side effects of NOCDURNA include:
- dry mouth
- low levels of salt in blood (hyponatremia)
- dizziness
These are not all of the possible side effects of NOCDURNA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store NOCDURNA?
Store NOCDURNA at room temperature between 68° to 77°F (20° to 25°C).
Keep NOCDURNA in its blister pack until it is time to take it, in order to protect it from moisture and light.
Keep NOCDURNA and all medicines out of the reach of children.
General information about the safe and effective use of NOCDURNA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NOCDURNA for a condition for which it was not prescribed.
Do not give NOCDURNA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NOCDURNA that is written for health professionals.
What are the ingredients in NOCDURNA?
Active ingredient: desmopressin acetate
Inactive ingredients: gelatin, NF (fish source), mannitol, anhydrous citric acid
Manufactured for: Antares Pharma, Inc. Ewing, NJ 08628 Origin Sweden
2009055856 LB-0153V0 11/2020
For more information, go to www.NOCDURNA.com or call 609-359-3020.
This Medication Guide has been approved by the U.S. Food and Drug Administration
11/2020
-
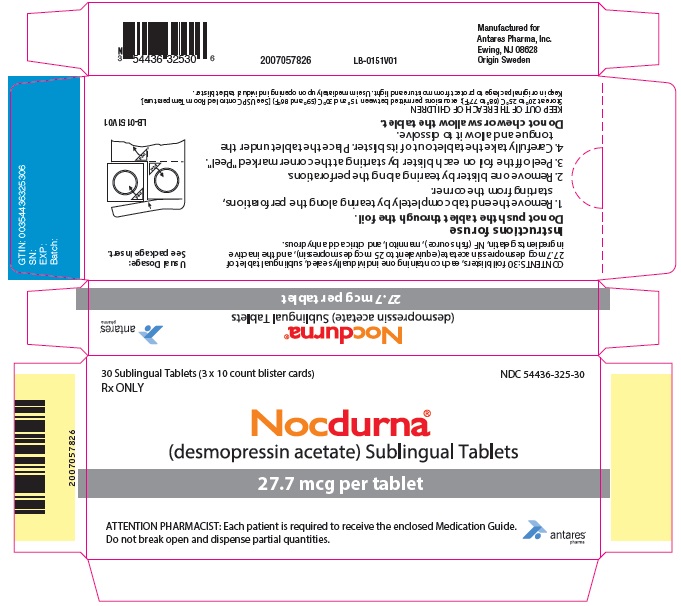
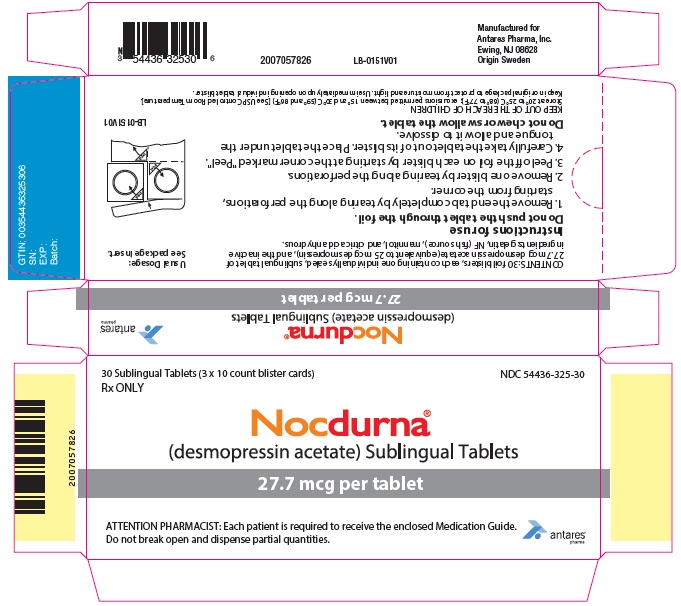
PRINCIPAL DISPLAY PANEL - 27.7 mcg Tablet Blister Pack Carton
30 Sublingual Tablets (3 x 10 count blister cards)
Rx ONLYNDC 54436-325-30
Nocdurna ®
(desmopressin acetate) Sublingual Tablets27.7 mcg per tablet
ATTENTION PHARMACIST: Each patient is required to receive the enclosed Medication Guide.
Do not break open and dispense partial quantities.Antares
Pharma
-
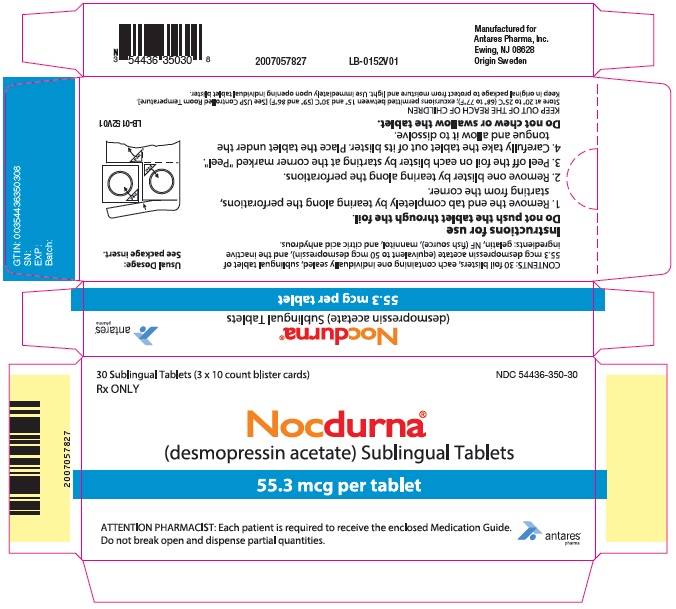
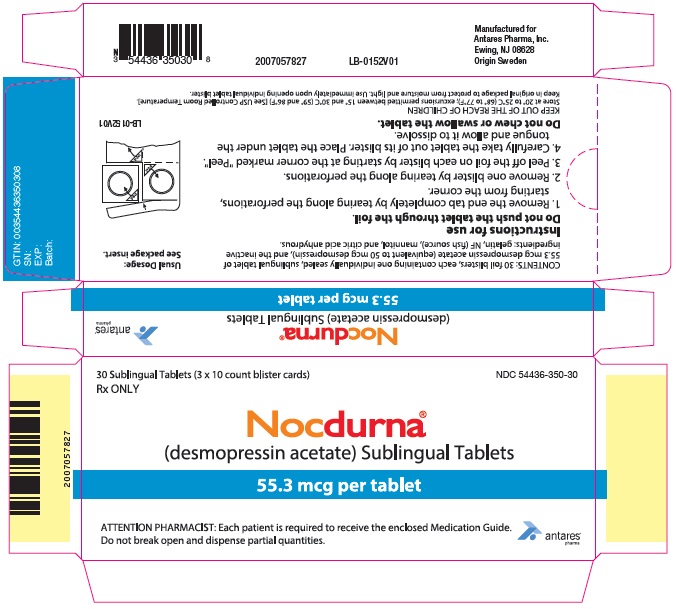
PRINCIPAL DISPLAY PANEL - 55.3 mcg Tablet Blister Pack Carton
30 Sublingual Tablets (3 x 10 count blister cards)
Rx ONLYNDC 54436-350-30
Nocdurna®
(desmopressin acetate) Sublingual Tablets55.3 mcg per tablet
ATTENTION PHARMACIST: Each patient is required to receive the enclosed Medication Guide.
Do not break open and dispense partial quantities.
Antares
Pharma
-
INGREDIENTS AND APPEARANCE
NOCDURNA
desmopressin acetate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-325 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DESMOPRESSIN ACETATE (UNII: XB13HYU18U) (DESMOPRESSIN - UNII:ENR1LLB0FP) DESMOPRESSIN ACETATE 27.7 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MARINE NON-GELLING GELATIN, HIGH MW (UNII: AHQ60JKI5D) Product Characteristics Color white Score no score Shape ROUND Size 14mm Flavor Imprint Code 25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-325-30 3 in 1 CARTON 08/06/2021 05/31/2025 1 NDC:54436-325-10 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022517 08/06/2021 05/31/2025 NOCDURNA
desmopressin acetate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-350 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DESMOPRESSIN ACETATE (UNII: XB13HYU18U) (DESMOPRESSIN - UNII:ENR1LLB0FP) DESMOPRESSIN ACETATE 55.3 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MARINE NON-GELLING GELATIN, HIGH MW (UNII: AHQ60JKI5D) Product Characteristics Color white Score no score Shape ROUND Size 14mm Flavor Imprint Code 50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-350-30 3 in 1 CARTON 08/06/2021 05/31/2025 1 NDC:54436-350-10 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022517 08/06/2021 05/31/2025 Labeler - Antares Pharma, Inc. (085369585) Establishment Name Address ID/FEI Business Operations Ferring Production Inc. 079510999 pack(54436-325, 54436-350) , label(54436-325, 54436-350) Establishment Name Address ID/FEI Business Operations Catalent Pharma Solutions Limited 237676320 manufacture(54436-325, 54436-350) , analysis(54436-325, 54436-350) Establishment Name Address ID/FEI Business Operations Ferring GmbH 328609615 analysis(54436-325, 54436-350) Establishment Name Address ID/FEI Business Operations Polypeptide Laboratories (Sweden) AB 356580779 api manufacture(54436-325, 54436-350) , ANALYSIS(54436-325, 54436-350) Establishment Name Address ID/FEI Business Operations Ferring International Center SA 481210362 pack(54436-325, 54436-350) , label(54436-350)