Label: CALCIUM GLUCONATE injection, solution
- NDC Code(s): 13985-802-50
- Packager: MWI/VetOne
- Category: OTC ANIMAL DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 27, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
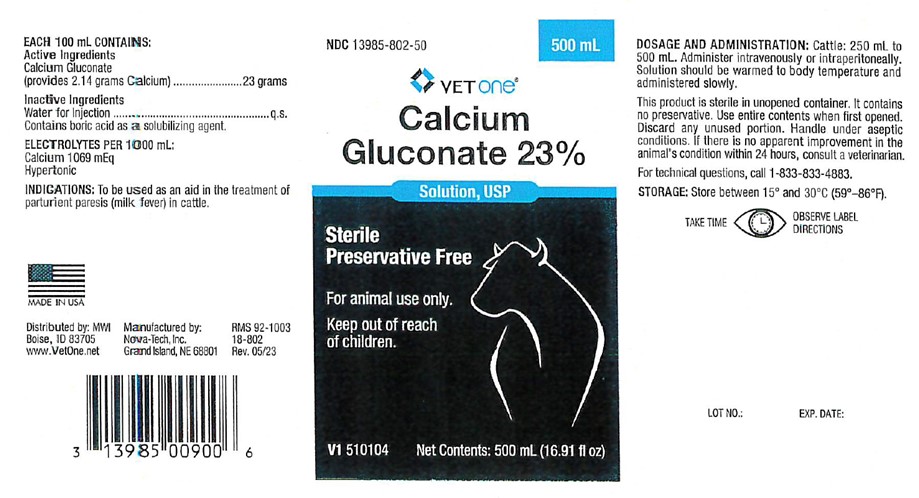
- EACH 100 mL CONTAINSActive Ingredients
- Inactive Ingredients
- INDICATIONS:
-
INFORMATION FOR OWNERS/CAREGIVERS
Distributed by: MWI
Boise, ID 83705
(888) 694-8381
www.VetOne.netManufactured by: Nova-Tech, Inc.
Grand Island, NE 68801Solution, USP
Sterile
Preservative FreeV1 510104 Net Contents: 500 mL
RMS 92-1003
18-802
Rev 05/23LOT NO.: EXP. DATE:
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE AND ADMINISTRATION:
Cattle: 250 mL to 500 mL. Administer intravenously or intraperitoneally. Solution should be warmed to body temperature and administered slowly.
This product is sterile in unopened container. It contains no preservative. Use entire contents when first opened. Discard any unused portion. Handle under aseptic conditions. If there is no apparent improvement in the animal's condition within 24 hours, consult a veterinarian.
- STORAGE:
- WARNINGS AND PRECAUTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALCIUM GLUCONATE
calcium gluconate injection, solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:13985-802 Route of Administration INTRAVENOUS, INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM GLUCONATE (UNII: SQE6VB453K) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM GLUCONATE 23 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-802-50 500 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/12/2014 Labeler - MWI/VetOne (019926120) Registrant - MWI/VetOne (019926120) Establishment Name Address ID/FEI Business Operations Nova-Tech, Inc. 196078976 manufacture