Label: SOLEIL SUPERIEUR BROAD SPECTRUM SPF 50 SUNSCREEN US- avobenzone, homosalate, octisalate, octocrylene cream
- NDC Code(s): 82691-146-00
- Packager: RV Skincare LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
• For sunscreen use: • apply liberally 15 minutes before sun exposure • use a water resistant sunscreen if swimming or sweating • reapply at least every 2 hours • Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. – 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses • Children under 6 months of age: Ask a doctor
Sun Protection Measures. - Other information
-
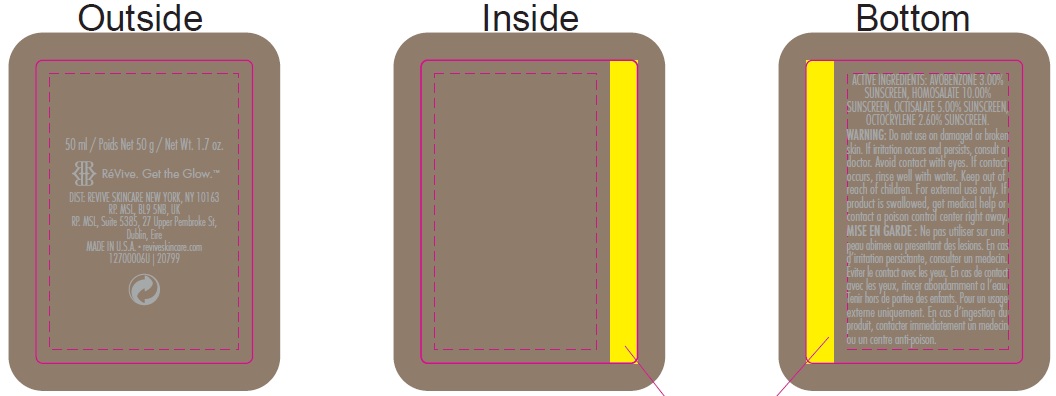
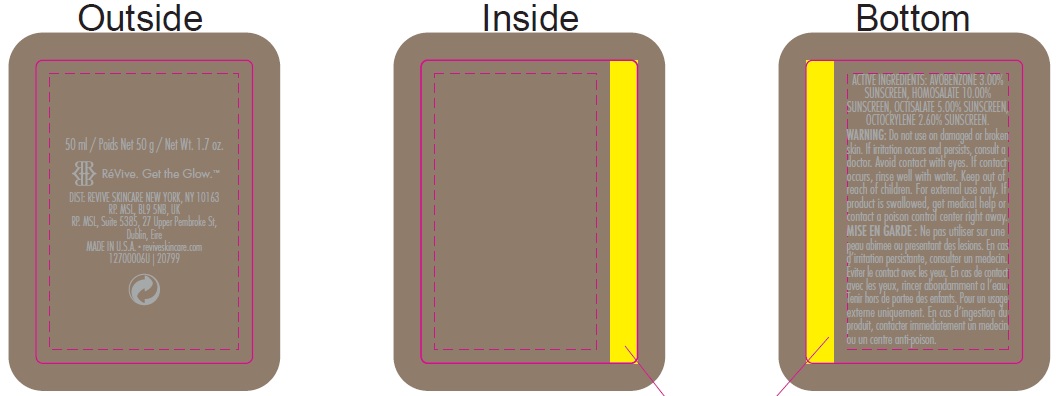
Inactive ingredients:
Aqua (Water, Eau), Dipropylene Glycol, Caprylyl Methicone, Dicaprylyl Carbonate, PEG/PPG-17/6 Copolymer, Potassium Cetyl Phosphate, Thermus Thermophillus Ferment, PEG-100 Stearate, Glyceryl Stearate, Methyl Glucose Sesquistearate, Alcohol Denat., Oligopeptide-24, Tocopheryl Acetate, Carnosine, Adenosine, Polygonum Aviculare Extract, Glycerin, Panthenol, Tocopherol, Sodium Oleate, Glycine Soja (Soybean) Oil, Hydrogenated Lecithin, Hydrogenated Palm Glycerides, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Cetyl Alcohol, Caprylyl Glycol, Isohexadecane, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Lecithin, Silybin, Potassium Sorbate, Potassium Hydroxide, Hexylene Glycol, Sorbitan Oleate, Parfum (Fragrance), Dipotassium Glycyrrhizate, C11-15 Alketh-7, C11-15 Alketh-40, Sodium Benzoate, Phenoxyethanol, Polyacrylate-15, Polyacrylate-17, Polysorbate 80, Disodium EDTA, PEG-10 Dimethicone, Disodium Lauryl Sulfosuccinate, Sodium Laureth-12 Sulfate.
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SOLEIL SUPERIEUR BROAD SPECTRUM SPF 50 SUNSCREEN US
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82691-146 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 26 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPROPYLENE GLYCOL (UNII: E107L85C40) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) PEG/PPG-17/6 COPOLYMER (UNII: P5QZM4T259) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYL GLUCOSE SESQUISTEARATE (UNII: V1YW10H14D) ALCOHOL (UNII: 3K9958V90M) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CARNOSINE (UNII: 8HO6PVN24W) ADENOSINE (UNII: K72T3FS567) POLYGONUM AVICULARE TOP (UNII: ZCD6009IUF) GLYCERIN (UNII: PDC6A3C0OX) PANTHENOL (UNII: WV9CM0O67Z) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM OLEATE (UNII: 399SL044HN) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED PALM GLYCERIDES (UNII: YCZ8EM144Q) CETYL ALCOHOL (UNII: 936JST6JCN) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISOHEXADECANE (UNII: 918X1OUF1E) SILIBININ (UNII: 4RKY41TBTF) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) SODIUM BENZOATE (UNII: OJ245FE5EU) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) DISODIUM LAURYL SULFOSUCCINATE (UNII: P160Q81342) SODIUM LAURETH-12 SULFATE (UNII: 8M492LDU23) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82691-146-00 1 in 1 CARTON 12/01/2017 1 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2017 Labeler - RV Skincare LLC (080986653)