Label: PLEO UT- bacillus subtilis liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 60681-5307-1, 60681-5307-2 - Packager: Sanum Kehlbeck GmbH & Co. KG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 5, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- INDICATIONS
- DIRECTIONS FOR USE
- DOSAGE
- INGREDIENTS
-
WARNING
If symptoms persist more than a few days, contact a licensed practitioner. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health care professional before using this product.
- SPL UNCLASSIFIED SECTION
-
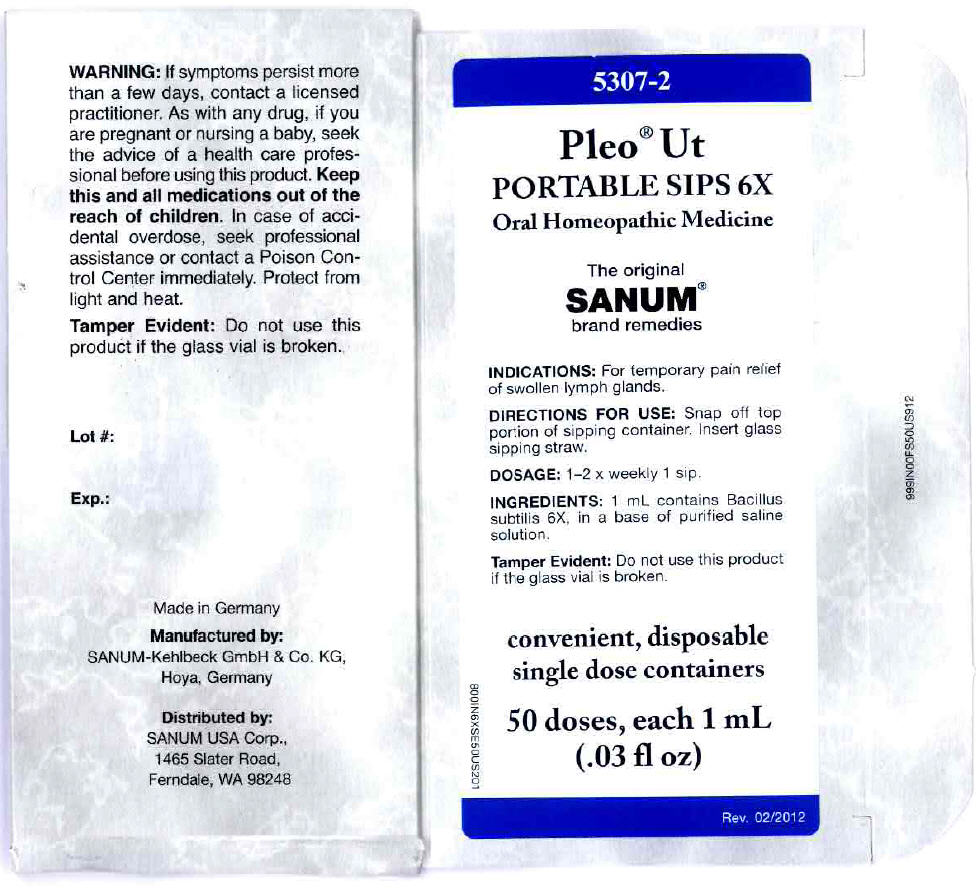
PRINCIPAL DISPLAY PANEL - 1 mL Vial Carton
5307-2
Pleo® Ut
PORTABLE SIPS 6XOral Homeopathic Medicine
The original
SANUM®
brand remediesINDICATIONS: For temporary pain relief
of swollen lymph glands.DIRECTIONS FOR USE: Snap off top
portion of sipping container. Insert glass
sipping straw.DOSAGE: 1–2 x weekly 1 sip.
INGREDIENTS: 1 mL contains Bacillus
subtilis 6X, in a base of purified saline
solution.Tamper Evident: Do not use this product
if the glass vial is broken.convenient, disposable
single dose containers50 doses, each 1 mL
(.03 fl oz)Rev. 02/2012
800IN6XSE50US201
-
INGREDIENTS AND APPEARANCE
PLEO UT
bacillus subtilis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60681-5307 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength bacillus subtilis (UNII: 8CF93KW41W) (bacillus subtilis - UNII:8CF93KW41W) bacillus subtilis 6 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) sodium chloride (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60681-5307-1 10 in 1 CARTON 1 1 mL in 1 VIAL, GLASS 2 NDC:60681-5307-2 50 in 1 CARTON 2 1 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 03/19/1997 Labeler - Sanum Kehlbeck GmbH & Co. KG (318386133)