Label: DAILY MOISTURIZING ANTIOXIDANT SPF 50- avobenzone, homosalate, octinoxate, octisalate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 10477-7720-1, 10477-7720-2, 10477-7720-3 - Packager: Goodier Cosmetics LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 19, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
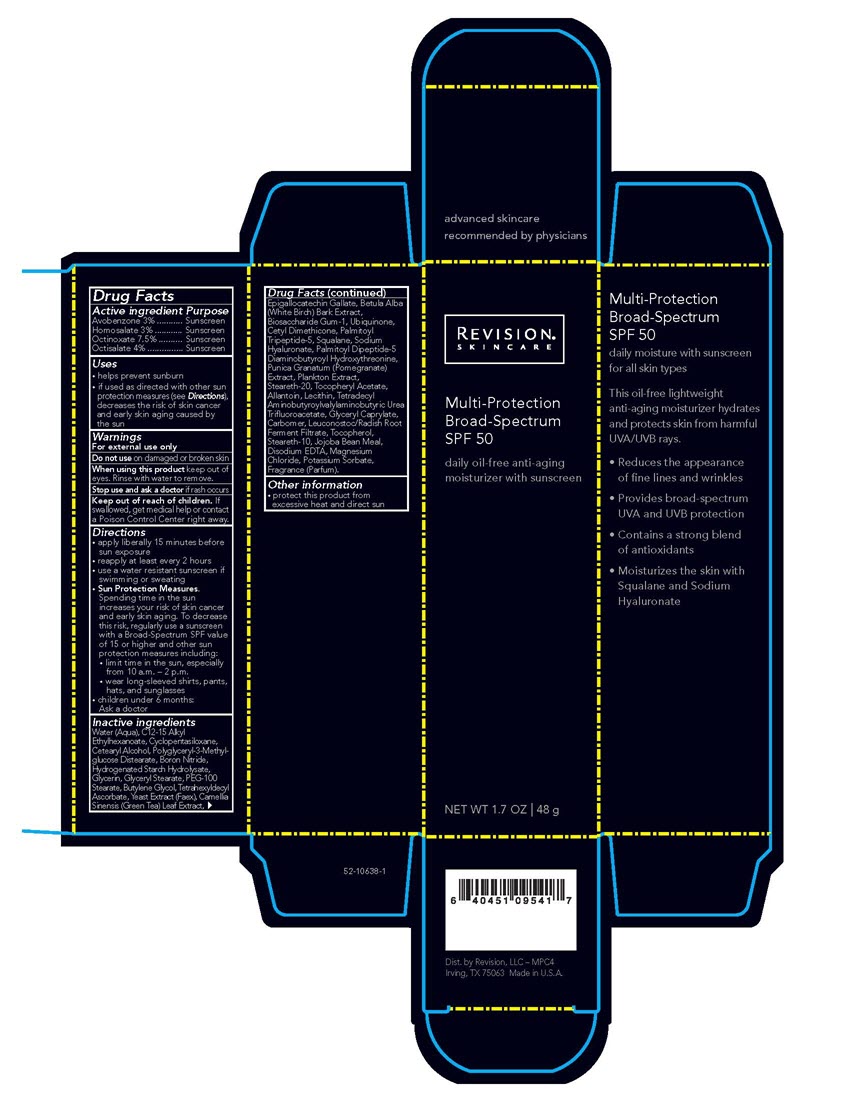
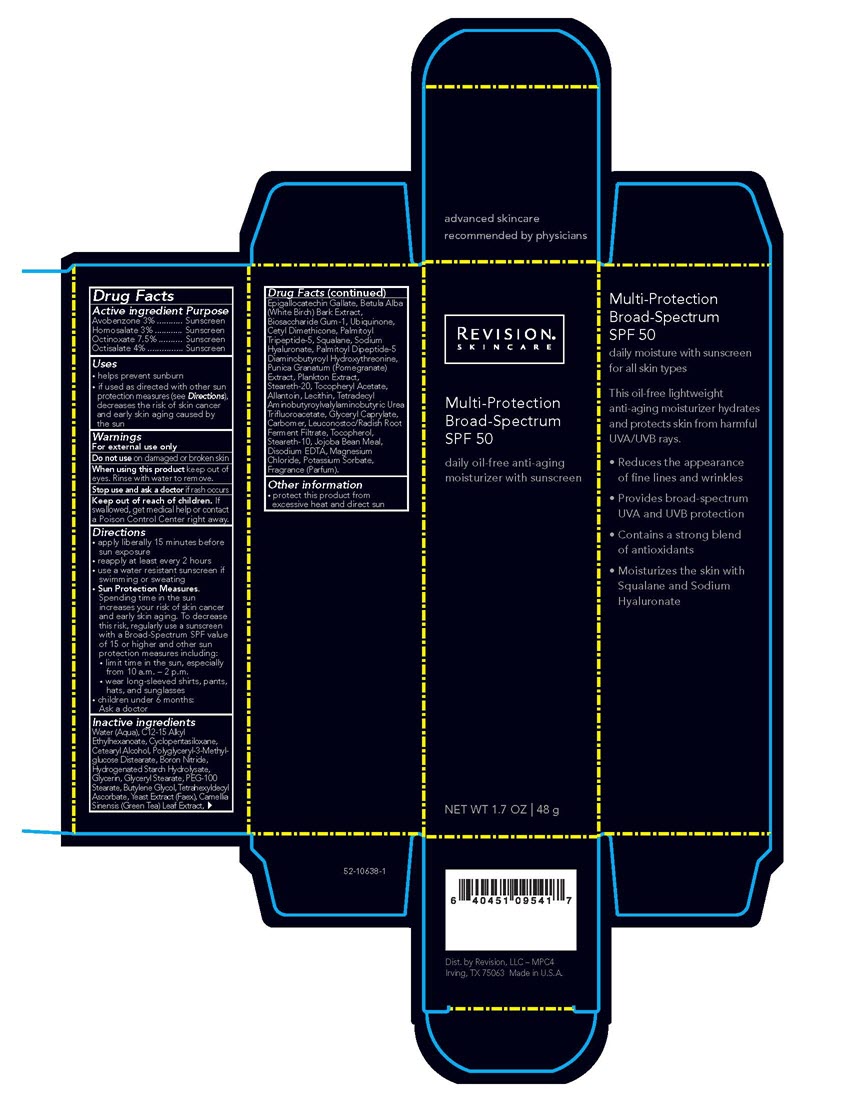
Revision Multi-Protection Broad-Spectrum SPF 50
Multi-Protection
Broad-Spectrum
SPF 50daily oil-free anti-aging
moisturizer with sunscreen
NET WT 1.7 OZ | 48 gActive ingredient Purpose
Avobenzone 3% ........... Sunscreen
Homosalate 3% ............ Sunscreen
Octinoxate 7.5% .......... Sunscreen
Octisalate 4% ............... SunscreenUses
• helps prevent sunburn
• if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sunDirections
• apply liberally 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen
with a Broad-Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months: Ask a doctorInactive ingredientsWater (Aqua), C12-15 Alkyl Ethylhexanoate, Cyclopentasiloxane, Cetearyl Alcohol, Polyglyceryl-3-Methylglucose Distearate, Boron Nitride,
Hydrogenated Starch Hydrolysate, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Butylene Glycol, Tetrahexyldecyl Ascorbate, Yeast Extract
(Faex), Camellia Sinensis (Green Tea) Leaf Extract, Epigallocatechin Gallate, Betula Alba (White Birch) Bark Extract, Biosaccharide Gum-1,
Ubiquinone, Cetyl Dimethicone, Palmitoyl Tripeptide-5, Squalane, Sodium Hyaluronate, Palmitoyl Dipeptide-5 Diaminobutyroyl
Hydroxythreonine, Punica Granatum (Pomegranate) Extract, Plankton Extract, Steareth-20, Tocopheryl Acetate, Allantoin, Lecithin,
Tetradecyl Aminobutyroylvalylaminobutyric Urea Trifluoroacetate, Glyceryl Caprylate, Carbomer, Leuconostoc/Radish Root Ferment Filtrate,
Tocopherol, Steareth-10, Jojoba Bean Meal, Disodium EDTA, Magnesium Chloride, Potassium Sorbate, Fragrance (Parfum). -
Revercel AM Moisturizing Sunscreen Broad Spectrum SPF 50
Active ingredient Purpose
Avobenzone 3% ........... Sunscreen
Homosalate 3% ............ Sunscreen
Octinoxate 7.5% .......... Sunscreen
Octisalate 4% ............... SunscreenUses
• helps prevent sunburn
• if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sunDirections
• apply liberally 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen
with a Broad-Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months: Ask a doctorInactive ingredients
Water (Aqua), C12-15 Alkyl Ethylhexanoate, Cyclopentasiloxane, Cetearyl Alcohol, Polyglyceryl-3-Methylglucose Distearate,
Boron Nitride, Sorbitol, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Butylene Glycol, Tetrahexyldecyl Ascorbate, Palmitoyl
Tripeptide-5, Glyceryl Caprylate, Leuconostoc/Radish Root Ferment Filtrate, Cetyl Dimethicone, Tocopherol, Squalane,
Steareth-20, Jojoba Bean Meal, Allantoin, Plankton Extract, Palmitoyl Dipeptide-5 Diaminobutyroyl Hydroxythreonine, Lecithin,
Sodium Hyaluronate, Betula Alba (White Birch) Bark Extract, Punica Granatum (Pomegranate) Extract, Yeast Extract (Faex),
Biosaccharide Gum-1, Tocopheryl Acetate, Tetradecyl Aminobutyroylvalylaminobutyric Urea Trifluoroacetate, Epigallocatechin
Gallate, Camellia Sinensis (Green Tea) Leaf Extract, Ubiquinone, Magnesium Chloride, Carbomer, Disodium EDTA, Steareth-10,
Potassium Sorbate, Fragrance (Parfum) - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DAILY MOISTURIZING ANTIOXIDANT SPF 50
avobenzone, homosalate, octinoxate, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10477-7720 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 3 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BORON NITRIDE (UNII: 2U4T60A6YD) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) YEAST (UNII: 3NY3SM6B8U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) EPIGALLOCATECHIN GALLATE (UNII: BQM438CTEL) BETULA PUBESCENS BARK (UNII: 3R504894L9) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) UBIDECARENONE (UNII: EJ27X76M46) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) PALMITOYL TRIPEPTIDE-5 (UNII: 2A3916MQHO) SQUALANE (UNII: GW89575KF9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PALMITOYLLYSYLVALYLDIAMINOBUTYROYLTHREONINE (UNII: 1615WE9073) POMEGRANATE (UNII: 56687D1Z4D) STEARETH-20 (UNII: L0Q8IK9E08) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALLANTOIN (UNII: 344S277G0Z) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) TOCOPHEROL (UNII: R0ZB2556P8) STEARETH-10 (UNII: FD0913P475) SIMMONDSIA CHINENSIS SEED (UNII: D24K2Q1F6H) EDETATE DISODIUM (UNII: 7FLD91C86K) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10477-7720-1 1 in 1 CARTON 06/26/2013 1 48 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10477-7720-2 227 g in 1 TUBE; Type 0: Not a Combination Product 06/26/2013 3 NDC:10477-7720-3 3 g in 1 TUBE; Type 0: Not a Combination Product 06/26/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/26/2013 Labeler - Goodier Cosmetics LLC (007317209) Registrant - Goodier Cosmetics LLC (007317209) Establishment Name Address ID/FEI Business Operations Goodier Cosmetics LLC 007317209 manufacture(10477-7720)