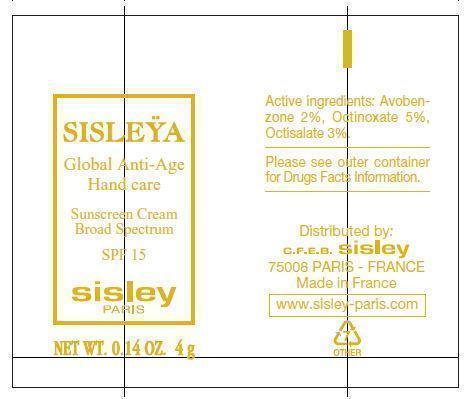
Label: SISLEYA GLOBAL ANTI-AGE HAND CARE SPF 15- avobenzone, octinoxate, octisalate cream
- NDC Code(s): 66097-001-04, 66097-001-76
- Packager: C.F.E.B. Sisley
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: ask a doctor
- Spending time in the sun increases our risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures.
- Limit time in the sun especially from 10 am to 2 pm
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Other information
-
Inactive ingredients
water, tribehenin peg-20 esters, sorbitol, isodecyl neopentanoate, butyrospermum parkii (shea butter), tropaeolum majus flower extract, helianthus annuus (sunflower) seed oil, glycyrrhiza glabra (licorice) root extract, harpagophytum procumbens root extract, cyclopentasiloxane, propylene glycol, phenoxyethanol, tocopheryl acetate, panthenol, pyrus malus (apple) seed extract, hexyldecanol, potassium cetyl phosphate, benzoic acid, sodium hydroxide, c12-13 pareth-23, methylparaben, aniba rosaeodora (rosewood) wood oil,edetate sodium, allantoin, c13-14 isoparaffin, retinyl palmitate, lavandula angustifolia (lavender) oil, pelargonium graveolens oil, caprylic/capric triglyceride, ethylparaben, citric acid, laureth-7, propylparaben, bht, ethylhexylglycerin, tocopherol, linalool, limonene, geraniol, citronellol, benzyl benzoate
- PRINCIPAL DISPLAY PANEL
- Product Labels
-
INGREDIENTS AND APPEARANCE
SISLEYA GLOBAL ANTI-AGE HAND CARE SPF 15
avobenzone, octinoxate, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66097-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 20 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 50 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TRIBEHENIN PEG-20 ESTERS (UNII: 84K9EH29Y9) SORBITOL (UNII: 506T60A25R) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) SHEANUT OIL (UNII: O88E196QRF) TROPAEOLUM MAJUS FLOWER (UNII: 7FV667B00B) SUNFLOWER OIL (UNII: 3W1JG795YI) GLYCERIN (UNII: PDC6A3C0OX) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) HARPAGOPHYTUM PROCUMBENS ROOT (UNII: 1OYM338E89) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PANTHENOL (UNII: WV9CM0O67Z) APPLE SEED (UNII: 33C88SEV0Z) HEXYLDECANOL (UNII: 151Z7P1317) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) BENZOIC ACID (UNII: 8SKN0B0MIM) SODIUM HYDROXIDE (UNII: 55X04QC32I) C12-13 PARETH-23 (UNII: J1WW1510L4) METHYLPARABEN (UNII: A2I8C7HI9T) ROSEWOOD OIL (UNII: F2522O5L7B) EDETATE SODIUM (UNII: MP1J8420LU) ALLANTOIN (UNII: 344S277G0Z) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) LAVENDER OIL (UNII: ZBP1YXW0H8) GERANIUM OIL, ALGERIAN TYPE (UNII: 5Q1I94P4WG) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ETHYLPARABEN (UNII: 14255EXE39) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) LAURETH-7 (UNII: Z95S6G8201) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TOCOPHEROL (UNII: R0ZB2556P8) CITRAL (UNII: T7EU0O9VPP) LINALOOL, (+/-)- (UNII: D81QY6I88E) GERANIOL (UNII: L837108USY) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) BENZYL BENZOATE (UNII: N863NB338G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66097-001-04 1 in 1 BOX 01/20/2013 1 4 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:66097-001-76 1 in 1 BOX 01/20/2013 2 76 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/20/2013 Labeler - C.F.E.B. Sisley (262279246)