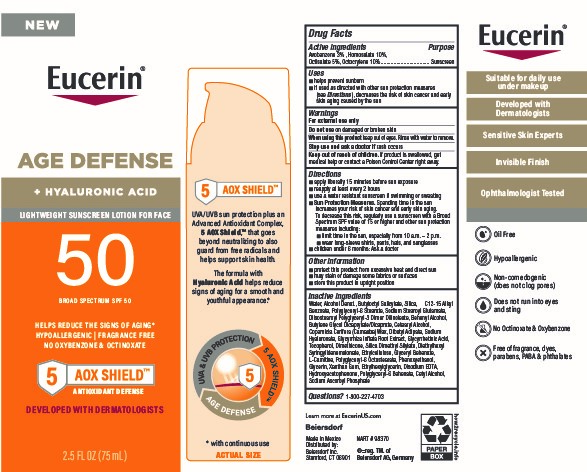
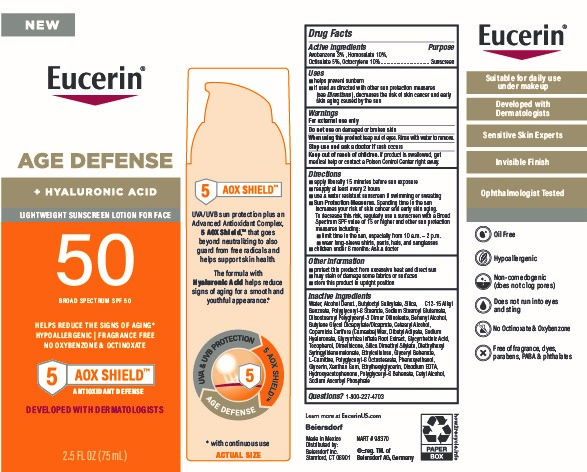
Label: EUCERIN AGE DEFENSE SPF 50 SUNSCREEN- avobenzone, homosalate, octisalate, octocrylene lotion
- NDC Code(s): 66800-5000-3, 66800-5000-4
- Packager: Beiersdort Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- DO NOT USE
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
-
Directions
■ apply liberally 15 minutes before sun exposure
■ reapply at least every 2 hours
■ use a water resistant sunscreen if swimming or sweating
■ Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
■ limit time in the sun, especially from 10 a.m. – 2 p.m.
■ wear long-sleeve shirts, pants, hats, and sunglasses
■ children under 6 months: Ask a doctor
- Other information
-
Inactive ingredients
Water, Alcohol Denat., Butyloctyl Salicylate, Silica, C12-15 Alkyl Benzoate, Polyglyceryl-6 Stearate, Sodium Stearoyl Glutamate, Diisostearoyl Polyglyceryl-3 Dimer Dilinoleate, Behenyl Alcohol, Butylene Glycol Dicaprylate/Dicaprate, Cetearyl Alcohol, Copernicia Cerifera (Carnauba) Wax, Dibutyl Adipate, Sodium Hyaluronate, Glycyrrhiza Inflata Root Extract, Glycyrrhetinic Acid, Tocopherol, Dimethicone, Silica Dimethyl Silylate, Diethylhexyl Syringylidenemalonate, Ethylcellulose, Glyceryl Behenate, L-Carnitine, Polyglyceryl-6 Octastearate, Phenoxyethanol, Glycerin, Xanthan Gum, Ethylhexylglycerin, Disodium EDTA, Hydroxyacetophenone, Polyglyceryl-6 Behenate, Cetyl Alcohol, Sodium Ascorbyl Phosphate
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EUCERIN AGE DEFENSE SPF 50 SUNSCREEN
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66800-5000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCINE PROPIONYL-L-CARNITINE (UNII: 8KVN59D5FL) POLYGLYCERYL-6 DISTEARATE (UNII: Z35I17EQOP) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM (UNII: 7FLD91C86K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) WATER (UNII: 059QF0KO0R) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ENOXOLONE (UNII: P540XA09DR) TOCOPHEROL (UNII: R0ZB2556P8) DIMETHICONE (UNII: 92RU3N3Y1O) DOCOSANOL (UNII: 9G1OE216XY) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) GLYCYRRHIZA INFLATA ROOT (UNII: 1MV1Z7MKVQ) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) POLYGLYCERYL-6 STEARATE (UNII: ETY9Q81E2T) DIISOSTEAROYL POLYGLYCERYL-3 DIMER DILINOLEATE (UNII: G3232Z5S2O) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) POLYGLYCERYL-6 BEHENATE (UNII: 4T2L7QI313) CETYL ALCOHOL (UNII: 936JST6JCN) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) ALCOHOL (UNII: 3K9958V90M) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) COPERNICIA PRUNIFERA WHOLE (UNII: 6EKZ38572S) DIBUTYL ADIPATE (UNII: F4K100DXP3) XANTHAN GUM (UNII: TTV12P4NEE) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) Product Characteristics Color white (Off-White to Yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66800-5000-4 75 g in 1 TUBE; Type 0: Not a Combination Product 11/03/2021 2 NDC:66800-5000-3 10 g in 1 POUCH; Type 0: Not a Combination Product 11/07/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/03/2021 Labeler - Beiersdort Inc (001177906)