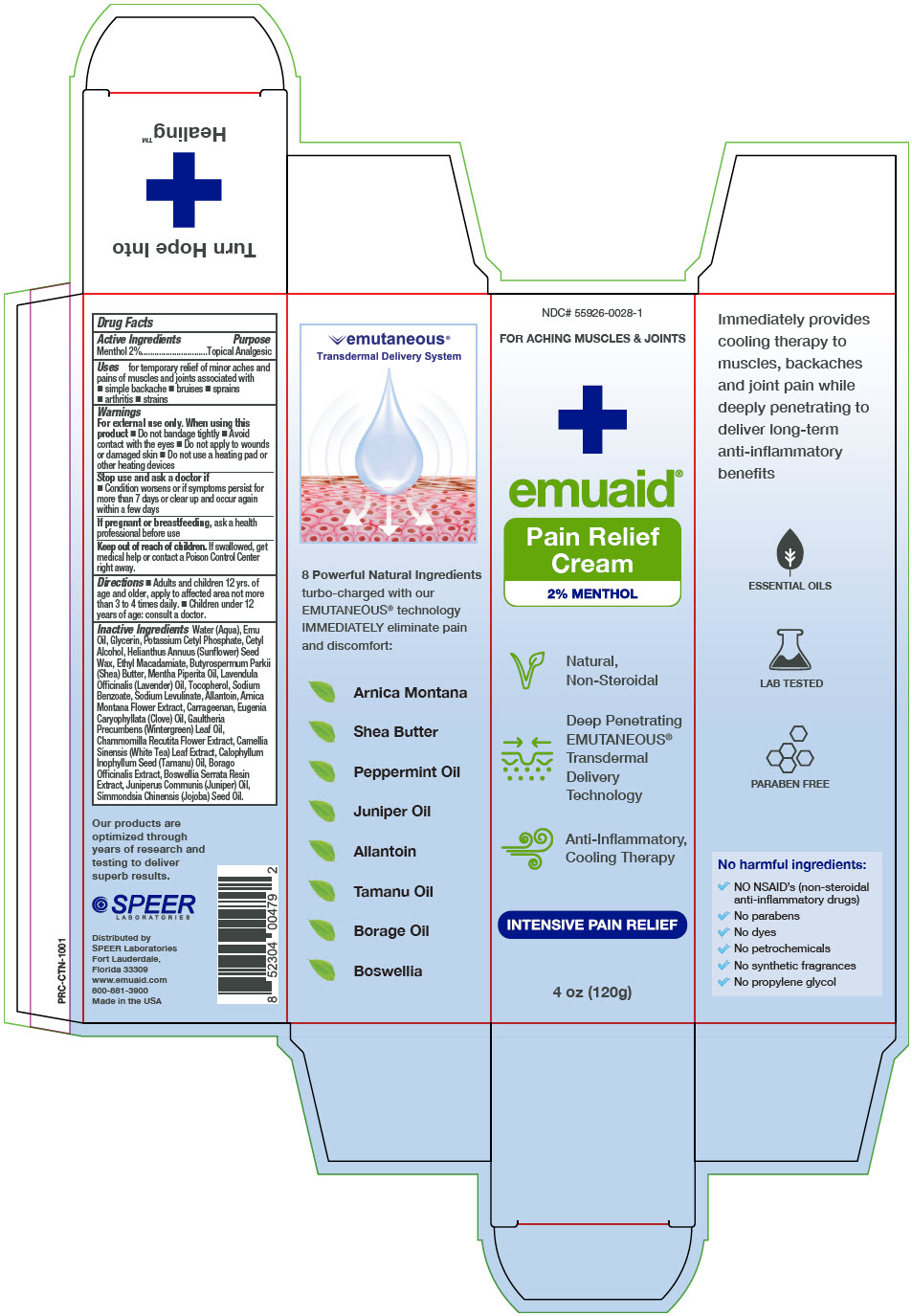
Label: EMUAID PAIN RELIEF- menthol cream
- NDC Code(s): 55926-0028-1
- Packager: Speer Laboratories LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only. When using this product
- Do not bandage tightly
- Avoid contact with the eyes
- Do not apply to wounds or damaged skin
- Do not use a heating pad or other heating devices
- Directions
-
Inactive Ingredients
Water (Aqua), Emu Oil, Glycerin, Potassium Cetyl Phosphate, Cetyl Alcohol, Helianthus Annuus (Sunflower) Seed Wax, Ethyl Macadamiate, Butyrospermum Parkii (Shea) Butter, Mentha Piperita Oil, Lavendula Officinalis (Lavender) Oil, Tocopherol, Sodium Benzoate, Sodium Levulinate, Allantoin, Arnica Montana Flower Extract, Carrageenan, Eugenia Caryophyllata (Clove) Oil, Gaultheria Precumbens (Wintergreen) Leaf Oil, Chammomilla Recutita Flower Extract, Camellia Sinensis (White Tea) Leaf Extract, Calophyllum Inophyllum Seed (Tamanu) Oil, Borago Officinalis Extract, Boswellia Serrata Resin Extract, Juniperus Communis (Juniper) Oil, Simmondsia Chinensis (Jojoba) Seed Oil.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 120 g Tube Box
-
INGREDIENTS AND APPEARANCE
EMUAID PAIN RELIEF
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55926-0028 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 2.4 g in 120 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EMU OIL (UNII: 344821WD61) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) CETYL ALCOHOL (UNII: 936JST6JCN) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) ETHYL MACADAMIATE (UNII: ANA2NCS6V1) SHEA BUTTER (UNII: K49155WL9Y) PEPPERMINT OIL (UNII: AV092KU4JH) LAVENDER OIL (UNII: ZBP1YXW0H8) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM LEVULINATE (UNII: VK44E1MQU8) ALLANTOIN (UNII: 344S277G0Z) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CARRAGEENAN (UNII: 5C69YCD2YJ) CLOVE OIL (UNII: 578389D6D0) METHYL SALICYLATE (UNII: LAV5U5022Y) CHAMOMILE (UNII: FGL3685T2X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TAMANU OIL (UNII: JT3LVK84A1) BORAGE (UNII: PB618V0K2W) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) JUNIPER BERRY OIL (UNII: SZH16H44UY) JOJOBA OIL (UNII: 724GKU717M) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55926-0028-1 1 in 1 BOX 11/14/2023 1 120 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 11/14/2023 Labeler - Speer Laboratories LLC (064900125) Establishment Name Address ID/FEI Business Operations Ariel Laboratories LP 087625133 MANUFACTURE(55926-0028)