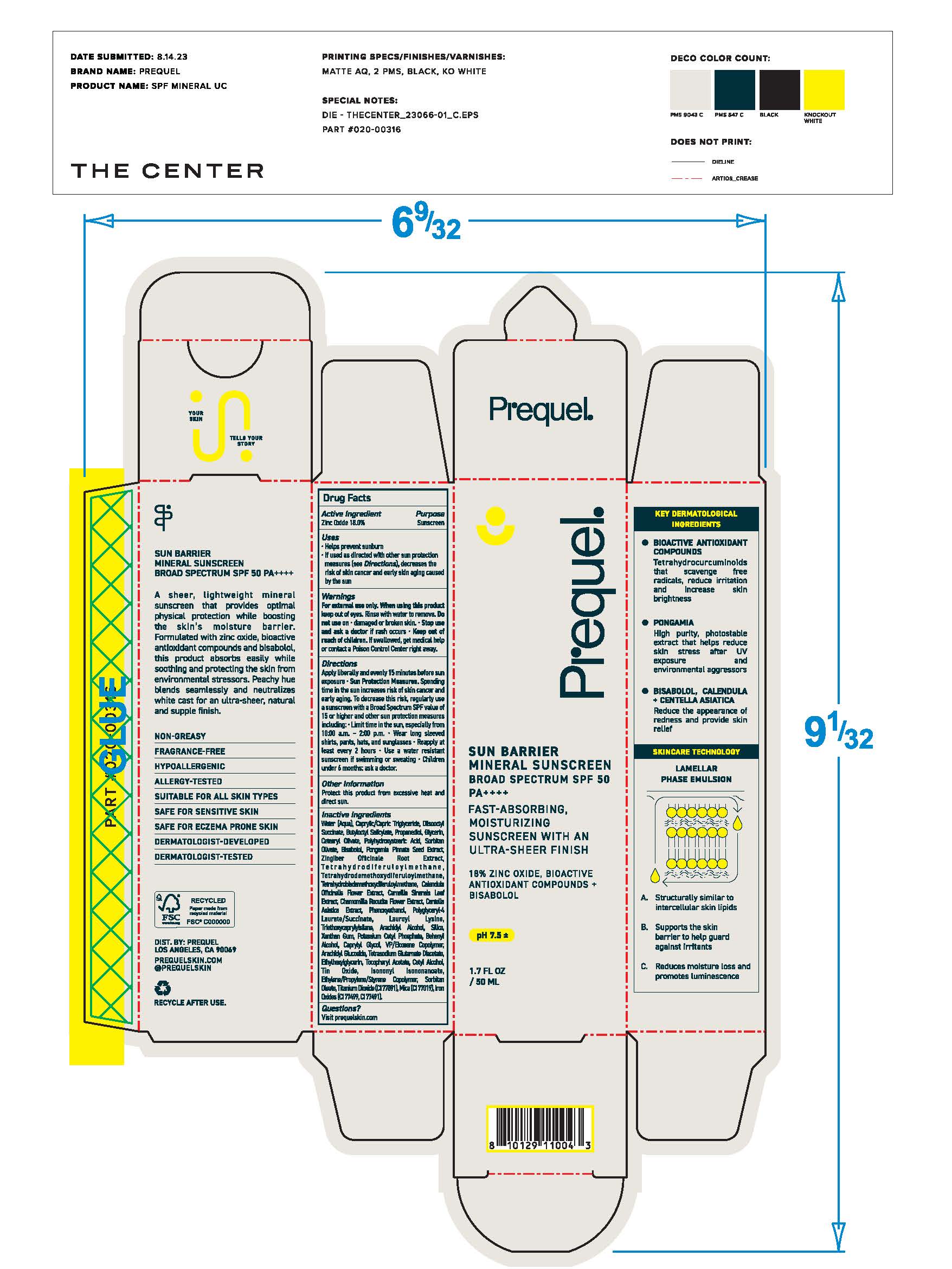
Label: PREQUEL SUN BARRIER MINERAL SUNSCREEN BROAD SPECTRUM SPF 50- mineral sunscreen cream
- NDC Code(s): 82800-010-01
- Packager: The Center Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Uses
- Warnings
-
Directions
• apply generously 15 minutes before sun exposure
• reapply: •at least every 2 hours.• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sunincreases your risk of skin cancer and early skin aging. To decrease
this risk, regularly use a sunscreen with a Broad Spectrum SPF
value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. - 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor -
Inactive ingredients
Water (Aqua), Caprylic/Capric Triglyceride, Diisooctyl Succinate, Butyloctyl Salicylate, Propanediol, Glycerin, Cetearyl Olivate, Polyhydroxystearic Acid, Sorbitan Olivate, Polyhydroxystearic Acid, Sorbitan Olivate, Bisabolol, Pongamia Pinnata Seed Extract, Zingiber Officinale Root Extract, Tetrahydrodiferuloylmethane, Tetrahydrodemethoxydiferuloylmethane, Tetrahydrodemethoxydiferuloylmethane, Calendula Officinalis Flower Extract, Camellia Sinensis Leaf Extract, Chamomilla Recutita Flower Extract, Centella Asiatica Extract, Phenoxyethanol, Polyglyceryl-4 Laurate/Succinate, Lauroyl Lysine, Triethoxycaprylylsilane, Arachidyl Alcohol, Silica, Xanthan Gum, Potassium Cetyl Phosphate, Behenyl Alcohol, Caprylyl Glycol, VP/Eicosene Copolymer, Arachidyl Glucoside, Tetrasodium Glutamate Diacetate, Ethylhexylglycerin, Tocopheryl Acetate, Cetyl Alcohol, Tin Oxide, Isononyl Isononanoate, Ethylene/Propylene/Styrene Copolymer, Soribitan Oleate, Titanium Dioxide (CI 77891), Mica (CI 77019), Iron Oxides (CI 77499, CI 77491)
- Other Information
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PREQUEL SUN BARRIER MINERAL SUNSCREEN BROAD SPECTRUM SPF 50
mineral sunscreen creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82800-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 18 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) DIISOCETYL ADIPATE (UNII: 14885YC9LU) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) CETEARYL OLIVATE (UNII: 58B69Q84JO) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SORBITAN OLIVATE (UNII: MDL271E3GR) .BETA.-BISABOLOL (UNII: LP618AV2EA) PONGAMIA PINNATA SEED (UNII: C2BRV53B1V) ZINGIBER OFFICINALE WHOLE (UNII: IN6Q3S3414) TETRAHYDRODIFERULOYLMETHANE (UNII: 00U0645U03) TETRAHYDRODEMETHOXYDIFERULOYLMETHANE (UNII: 44D8X9U00T) TETRAHYDROBISDEMETHOXYDIFERULOYLMETHANE (UNII: 973IBV8W7I) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CAMELLIA SINENSIS SEED (UNII: MRL49Z8AC4) MATRICARIA CHAMOMILLA FLOWERING TOP (UNII: 3VNC7T6Z02) CENTELLA ASIATICA (UNII: 7M867G6T1U) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) LAUROYL LYSINE (UNII: 113171Q70B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) SILICON (UNII: Z4152N8IUI) XANTHAN GUM (UNII: TTV12P4NEE) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) CAPRYLYL GLYCOL (UNII: 00YIU5438U) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CETYL ALCOHOL (UNII: 936JST6JCN) STANNIC OXIDE (UNII: KM7N50LOS6) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MICA (UNII: V8A1AW0880) BROWN IRON OXIDE (UNII: 1N032N7MFO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82800-010-01 1 in 1 PACKAGE 09/21/2023 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 09/21/2023 Labeler - The Center Brands, LLC (076228814)