Label: OB COMPLETE PREMIER- .beta.-carotene, calcium ascorbate, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium malate, iron, ferrous asparto glycinate, magnesium malate, zinc glycinate, and copper tablet, coated
- NHRIC Code(s): 68025-043-30
- Packager: Vertical Pharmaceuticals, LLC
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated February 18, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SUPPLEMENT FACTS
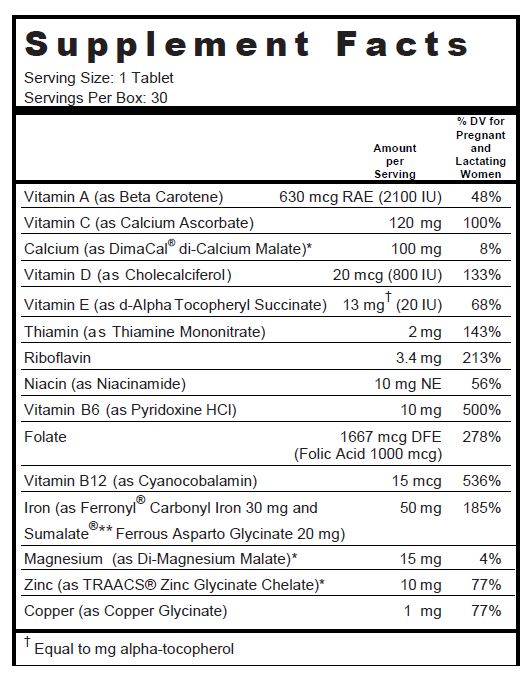
Other Ingredients: Microcrystalline Cellulose, DiCal Phosphate, Croscarmellose Sodium, Pink Color Coating (Hydroxypropylmethyl Cellulose, Polyvinyl Alcohol, Titanium Dioxide, Polyethylene Glycol, Talc and FDC Red #40 Lake), Fumed Silica, Stearic Acid, Magnesium Stearate, Acacia and Povidone K30.
VEGETARIAN FRIENDLY
GLUTEN-, LACTOSE- AND SUGAR-FREE
OB Complete® Premier is a prescription multivitamin/multimineral indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and non-lactating mothers. - CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered. -
DRUG INTERACTIONS
OB Complete® Premier tablets are not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
-
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained herein. However, allergic and idiosyncratic reactions are possible at lower levels. Iron, even at the usual recommended levels, has been associated with gastrointestinal intolerance in some patients.
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
-
HEALTH CLAIM
KEEP THIS PRODUCT OUT OF REACH OF CHILDREN.
*DimaCal and TRAACS are registered trademarks of Albion International, Inc. Malates covered by U.S. Patent No. 6,706,904. Chelate covered by U.S. Patent No. 7,838,042 and patents pending.
**Sumalate® (Ferrous Asparto Glycinate) is a registered trademark of Albion International, Inc. and is covered by the following patents: U.S. Pat. No. 6,716,814; U.S. Pat. No. 8,007,846 and U.S. Pat. No. 8,425,956.
For use on the order of a healthcare practitioner
Call your doctor about side effects. To report side effects, call Vertical Pharmaceuticals at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PLR-OBCPR-00005-2 Rev. 02/2022
Distributed by:
Vertical Pharmaceuticals, LLC
Alpharetta, GA 30005
- PRINCIPAL DISPLAY PANEL - 30 Tablet Blister Pack Carton
-
INGREDIENTS AND APPEARANCE
OB COMPLETE PREMIER
.beta.-carotene, calcium ascorbate, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium malate, iron, ferrous asparto glycinate, magnesium malate, zinc glycinate, and copper tablet, coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:68025-043 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETA CAROTENE (UNII: 01YAE03M7J) (BETA CAROTENE - UNII:01YAE03M7J) BETA CAROTENE 2100 [iU] CALCIUM ASCORBATE (UNII: 183E4W213W) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 800 [iU] .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 20 [iU] THIAMINE MONONITRATE (UNII: 8K0I04919X) (Thiamine ION - UNII:4ABT0J945J) THIAMINE 2 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3.4 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 10 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 10 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 15 ug CALCIUM MALATE (UNII: D48OP746DW) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 100 mg IRON PENTACARBONYL (UNII: 6WQ62TAQ6Z) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 30 mg FERROUS ASPARTO GLYCINATE (UNII: H7426RGB3L) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 20 mg MAGNESIUM MALATE (UNII: LA9X9UJA2Z) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 15 mg ZINC GLYCINATE (UNII: 681VJX72FE) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 10 mg CUPRIC GLYCINATE (UNII: 68VAV8QID7) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 1 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CALCIUM PHOSPHATE (UNII: 97Z1WI3NDX) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FD&C RED NO. 40 (UNII: WZB9127XOA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) ACACIA (UNII: 5C5403N26O) POVIDONE K30 (UNII: U725QWY32X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:68025-043-30 3 in 1 CARTON 1 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 12/31/2010 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color imprint scoring 1 shape size (solid drugs) 19 mm Labeler - Vertical Pharmaceuticals, LLC (173169017)


