Label: XDEMVY- lotilaner ophthalmic solution solution/ drops
- NDC Code(s): 81942-125-01, 81942-125-99
- Packager: Tarsus Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XDEMVY safely and effectively. See full prescribing information for XDEMVY.
XDEMVY™ (lotilaner ophthalmic solution) 0.25%, for topical ophthalmic use
Initial U.S. Approval: 2023INDICATIONS AND USAGE
XDEMVY is an ectoparasiticide (anti-parasitic) indicated for the treatment of Demodex blepharitis. ( 1)
DOSAGE AND ADMINISTRATION
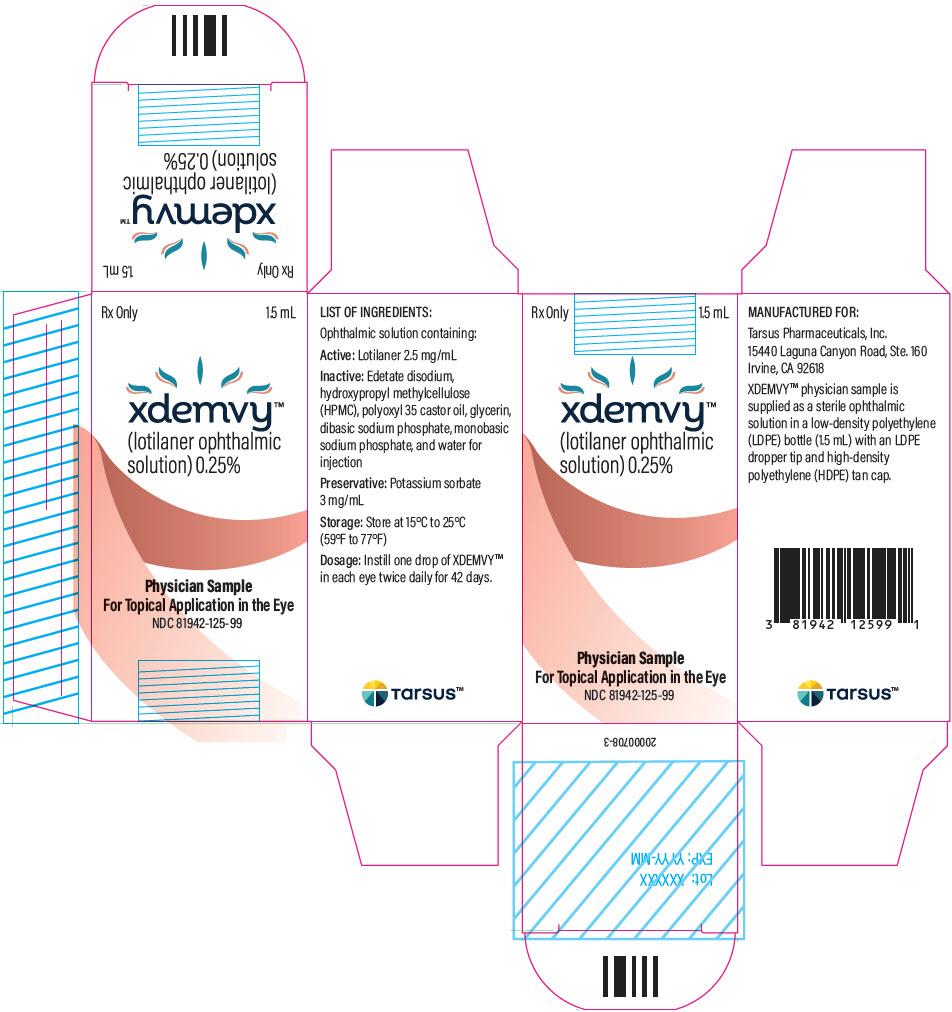
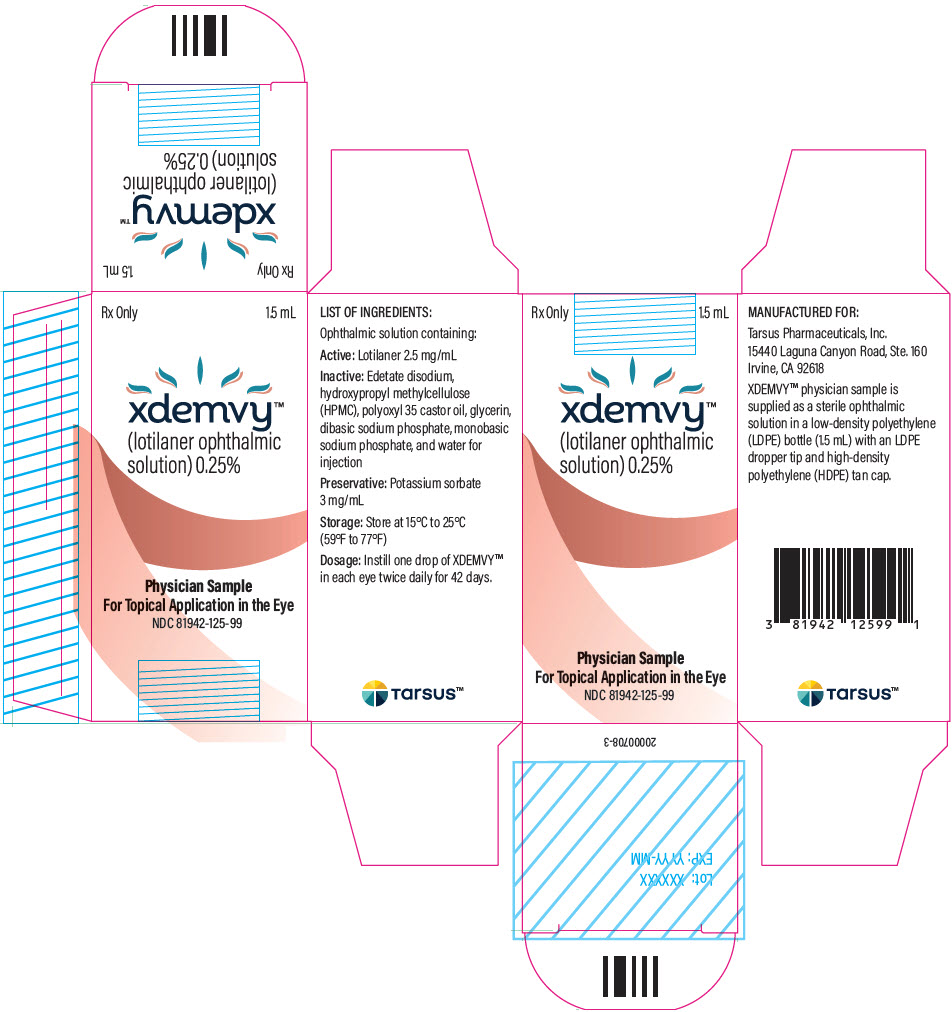
Instill one drop of XDEMVY in each eye twice daily (approximately 12 hours apart) for 6 weeks. ( 2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing lotilaner 0.25%. ( 3)
CONTRAINDICATIONS
None. ( 4)
ADVERSE REACTIONS
The most common adverse reaction was instillation site stinging and burning (10%). ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Tarsus Pharmaceuticals at 1-888-421-4002 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Contamination
5.2 Use with Contact Lenses
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Contamination
Do not allow the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to minimize contamination of the solution. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions .
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
XDEMVY was evaluated in 833 patients with Demodex blepharitis in two randomized, double-masked, vehicle-controlled studies (Saturn-1 and Saturn-2) with 42 days of treatment. The most common ocular adverse reaction observed in controlled clinical studies with XDEMVY was instillation site stinging and burning which was reported in 10% of patients. Other ocular adverse reactions reported in less than 2% of patients were chalazion/hordeolum and punctate keratitis.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on XDEMVY use in pregnant women to inform any drug associated risk; however, systemic exposure to lotilaner from ocular administration is low [see Clinical Pharmacology (12.3)] . In animal reproduction studies, lotilaner did not produce malformations at clinically relevant doses.
Data
Animal Data
In an oral embryofetal developmental study in pregnant rats dosed during organogenesis from gestation days 6-19, increased post-implantation loss, reduced fetal pup weight, and incomplete skeletal ossification were observed at 50 mg/kg/day (approximately 1390 times the recommended human ophthalmic dose (RHOD) on a body surface area basis) in the presence of maternal toxicity (i.e., decreased body weight and food consumption). A rare malformation of situs inversus of the thoracic and abdominal viscera occurred in 1 fetus from a pregnant rat receiving 50 mg/kg/day; whether this finding was treatment-related could not be excluded. No maternal or embryofetal toxicity was observed at 18 mg/kg/day (approximately 501 times the RHOD on a body surface area basis). In an oral embryofetal development study in pregnant rabbits dosed during organogenesis from gestation days 7-19, no embryofetal toxicity or teratogenic findings were observed at 20 mg/kg/day (approximately 580-times the RHOD on an AUC basis), even in the presence of maternal toxicity (i.e., decreased food consumption and body weight).
In an oral two-generation reproductive toxicity study, F0 male and female rats were administered lotilaner at doses up to 40 mg/kg/day for 10 weeks before pairing and during the 2-week pairing period (3 weeks for males). Dosing for F0 females continued through lactation day 22. F1 male and female rats were administered lotilaner at 1 and 5 mg/kg/day post-weaning from day 23 for 10 weeks before pairing and during the 2-week pairing period (3 weeks for males). Dosing for F1 parenteral females continued through lactation day 22. There were no clear adverse effects on the F1 generation, and a slightly lower mean body weight during lactation was noted for F2 pups at 5 mg/kg/day. The no observed adverse effect level (NOAEL) was determined to be 5 mg/kg/day (approximately 139 times the RHOD on a body surface area basis).
8.2 Lactation
Risk Summary
There are no data on the presence of XDEMVY in human milk, the effects on the breastfed infant, or the effects on milk production. However, systemic exposure to lotilaner following 6 weeks of topical ocular administration is low and is >99% plasma protein bound [see Clinical Pharmacology (12.3)] , thus it is not known whether measurable levels of lotilaner would be present in maternal milk following topical ocular administration. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for XDEMVY and any potential adverse effects on the breast-fed child from XDEMVY.
-
11 DESCRIPTION
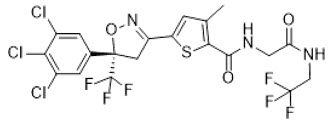
Lotilaner is a member of the isoxazoline family of compounds. Its chemical name is 2-Thiophenecarboxamide, 5-[(5S)-4,5-dihydro-5-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-3-isoxazolyl]-3-methyl-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-2-thiophenecarboxamide. The molecular formula is C 20H 14Cl 3F 6N 3O 3S. The molecular weight is 596.76 g/mol. The chemical structure is:
XDEMVY is a sterile, preserved, multi-dose, slightly yellowish, slightly opalescent, topical ophthalmic solution containing lotilaner, 0.25% as the active ingredient. It is preserved with potassium sorbate and contains the following additional inactive ingredients: edetate disodium, hydroxypropyl methylcellulose (HPMC), polyoxyl 35 castor oil, glycerin, dibasic sodium phosphate, monobasic sodium phosphate, and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lotilaner is a gamma-aminobutyric acid (GABA)-gated chloride channel inhibitor selective for mites. Inhibition of these GABA chloride channels causes a paralytic action in the target organism leading to its death. Lotilaner is not an inhibitor of mammalian GABA mediated chloride channels when tested at up to 30 µM (18 µg/mL) in vitro(approximately 1100 times the RHOD).
12.3 Pharmacokinetics
The systemic pharmacokinetic profile after topical ocular administration was evaluated in healthy volunteers after single and repeat dose administration. The systemic exposure was evaluated in patients at the end of 6 weeks of treatment.
Absorption
Maximum lotilaner concentration was observed 2 hours after a single ocular administration on Day 1 and 1 hour after the last drug administration on Day 42. In healthy subjects, the peak concentration (C max) and total exposure (AUC 0-12) of lotilaner in whole blood increased after 42 days of repeated ocular administration from 0.596 to 17.8 ng/mL and from 5.75 to 149 hr•ng/mL for C maxand AUC 0-12respectively. The effective half-life of lotilaner, which is based on the accumulation ratio over the dosing interval of 12 hours, was 264 hours (11 days). In patients with Demodex blepharitis (n=138) who received XDEMVY twice daily for 42 days, the mean (range) systemic exposure at the end of treatment was 12.0 ng/mL (0.4-46.1 ng/mL).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of lotilaner.
Mutagenesis
Lotilaner was not genotoxic in the following assays: Ames assay for bacterial gene mutation, in vitrochromosomal aberration assay in cultured human peripheral blood lymphocytes, and in vivorat micronucleus test.
Impairment of fertility
In a two-generation study of reproductive performance in rats, F0 male and female rats were administered lotilaner at oral doses of 40 mg/kg/day for 80 days reduced to 20 mg/kg/day for 47-50 supplementary days. Reduced pregnancy rates and decreased implantation rates were observed in F0 females at doses 20 mg/kg/day) (approximately 556 times the RHOD on a body surface area basis), which were also associated with maternal toxicity (i.e., decreased body weight and food consumption). No effects on fertility were observed in F0 females at the dose of 5 mg/kg/day (approximately 139 times the MRHOD on a body surface area basis) .No effects on fertility were observed in F0 males at the oral dose of 20 mg/kg/day (approximately 556 times the RHOD on a body surface area basis), and no effects on fertility were observed in F1 males and females at the oral dose of 5 mg/kg/day (approximately 139 times the RHOD on a body surface area basis).
-
14 CLINICAL STUDIES
The safety and efficacy of XDEMVY for the treatment of Demodex blepharitis was evaluated in a total of 833 patients (415 of which received XDEMVY) in two 6-week, randomized, multicenter, double-masked, vehicle-controlled studies (Saturn-1 and Saturn-2). Patients with Demodex blepharitis were randomized to either XDEMVY or Vehicle at a 1:1 ratio dosed twice daily in each eye.
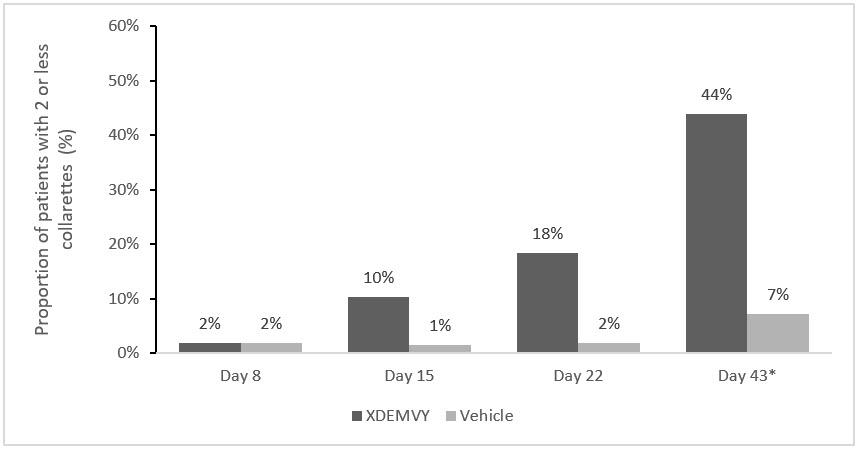
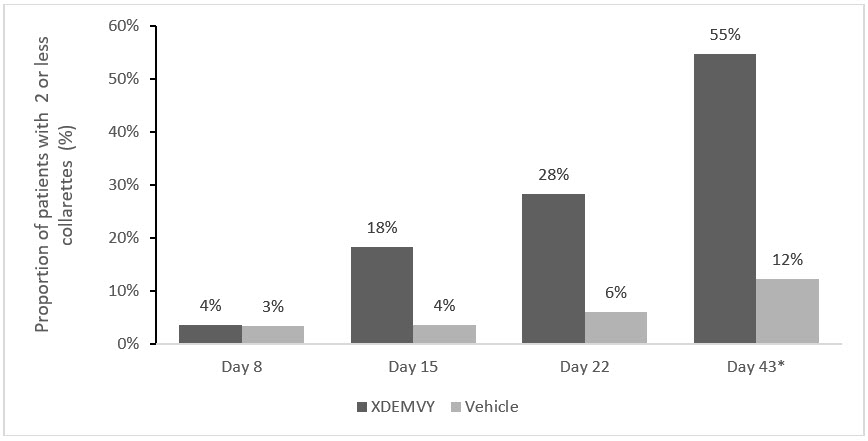
Efficacy was demonstrated by improvement in lids (reduction of collarettes to no more than 2 collarettes per upper lid) in each study (Saturn-1 and Saturn-2) (see Figure 1and Figure 2) by Day 43.
Figure 1. Saturn-1: Proportion of patients with 2 or less collarettes for the upper eyelid
*Day 43 Primary Endpoint; XDEMVY N=209, Vehicle N=204, p-value <0.01
Figure 2. Saturn-2: Proportion of patients with 2 or less collarettes for the upper eyelid
*Day 43 Primary Endpoint; XDEMVY N=193, Vehicle N=200, p-value <0.01
The endpoints of mite eradication (mite density of 0 mites/lash) and erythema cure (Grade 0) of XDEMVY vs. Vehicle demonstrated statistically significant improvement at Day 43 across both Saturn-1 (Table 1) and Saturn-2 (Table 2) studies.
Table 1: Proportion of patients with eradication of Demodex mites and erythema cure in the analysis eye at Day 43 in Saturn-1 XDEMVY (N=212) Vehicle (N=209) p-value Mite Eradication 68% 17% < 0.01 Erythema Cure 19% 7% < 0.01 Table 2: Proportion of patients with eradication of Demodex mites and erythema cure in the analysis eye at Day 43 in Saturn-2 XDEMVY (N=203) Vehicle (N=209) p-value Mite Eradication 50% 14% < 0.01 Erythema Cure 30% 9% < 0.01 - 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Handling the Container
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to minimize contamination of the solution. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions .
When to Seek Physician Advice
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection), have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician's advice concerning the continued use of XDEMVY.
Use with Contact Lenses
Advise patients that XDEMVY contains potassium sorbate, which may discolor soft contact lenses. Contact lenses should be removed prior to instillation of XDEMVY and may be reinserted 15 minutes following its administration.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1.5 mL Bottle Carton
- PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
XDEMVY
lotilaner ophthalmic solution solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81942-125 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOTILANER (UNII: HEH4938D7K) (LOTILANER - UNII:HEH4938D7K) LOTILANER 2.5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81942-125-01 1 in 1 CARTON 08/14/2023 1 10 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:81942-125-99 1 in 1 CARTON 08/14/2023 2 1.5 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217603 08/14/2023 Labeler - Tarsus Pharmaceuticals, Inc. (081222566)