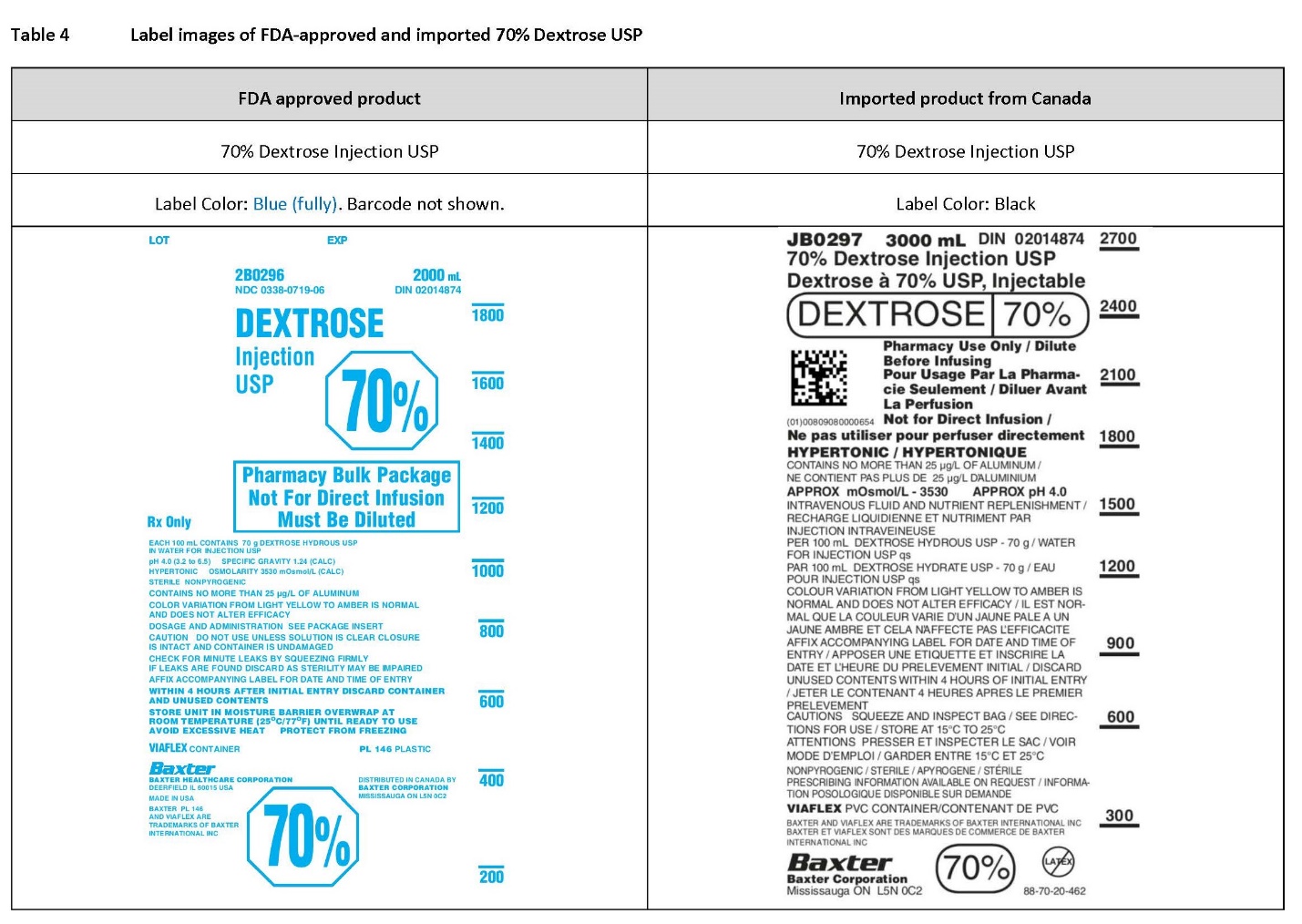
Label: DEXTROSE- dextrose monohydrate injection, solution
- NDC Code(s): 0338-9789-01, 0338-9789-04
- Packager: Baxter Healthcare Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Health Care Provider Letter
-
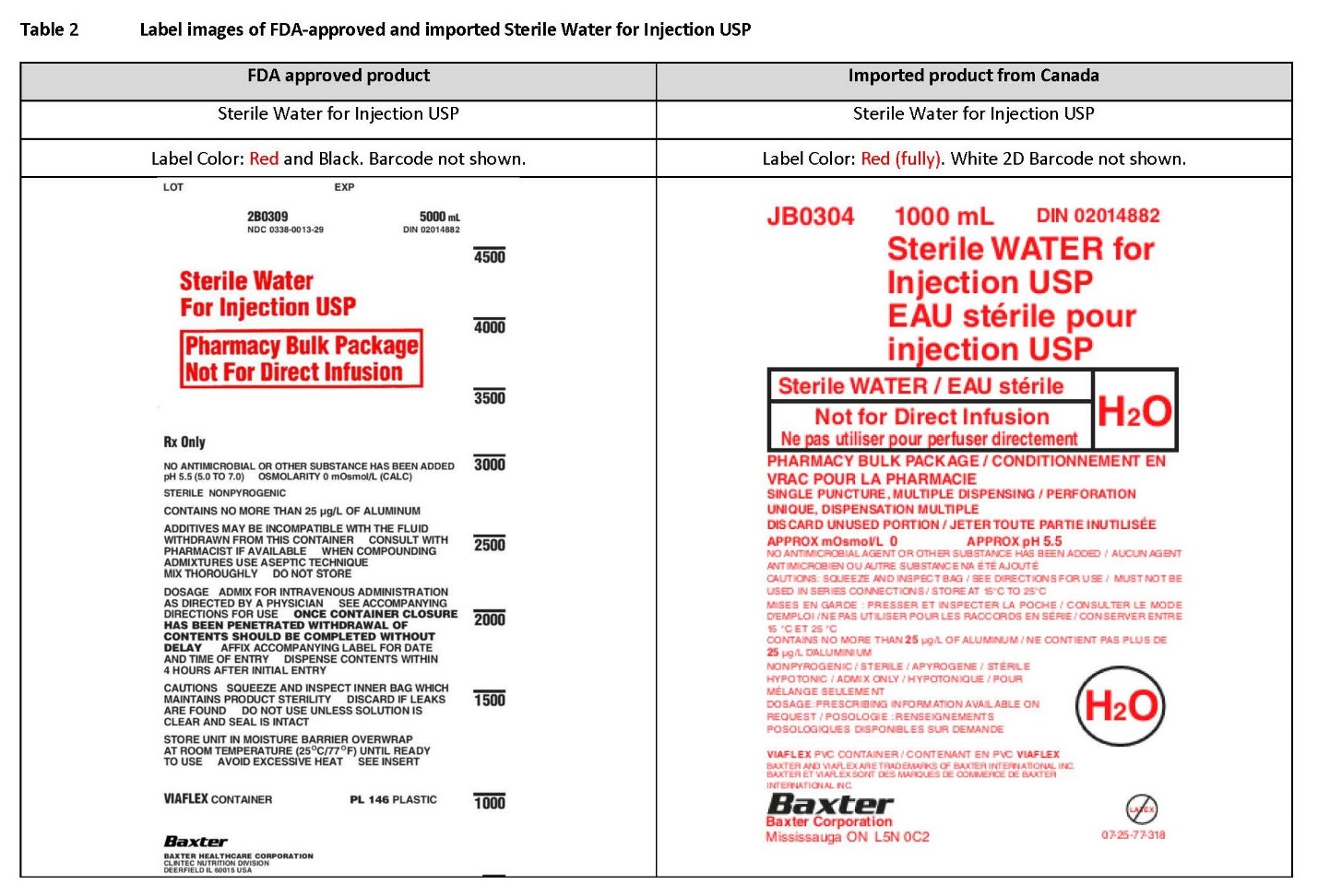
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Container Label
JB0297 3000 mL DIN 02014874
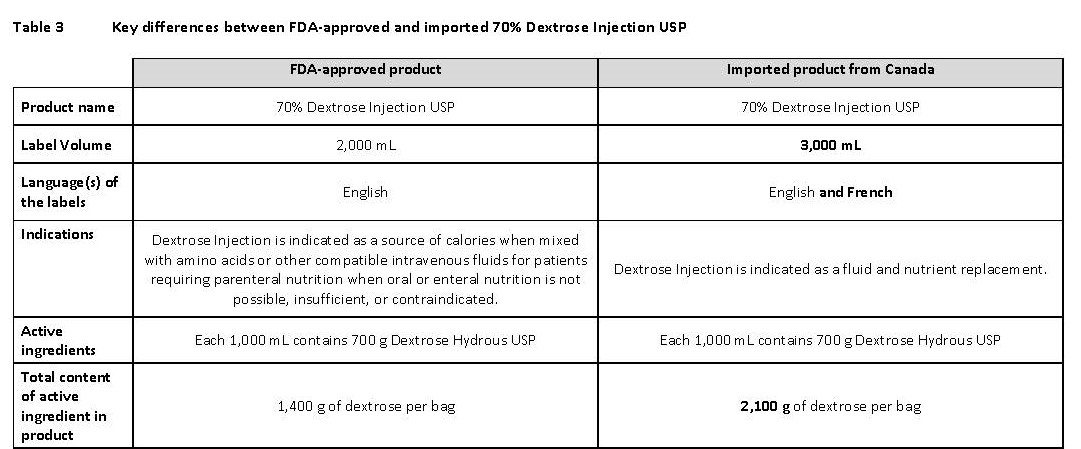
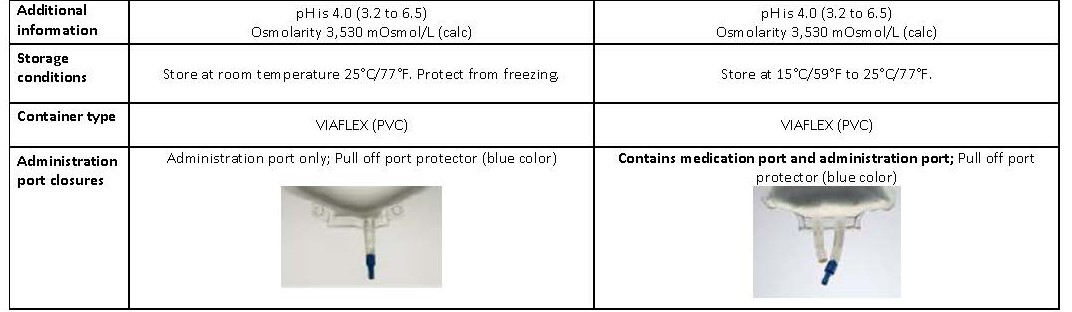
70% Dextrose Injection USP
Dextrose à 70% USP, InjectableDEXTROSE 70%
2D Barcode
(01)00809080000654Pharmacy Use Only / Dilute
Before Infusing
Pour Usage Par La Pharma-
cie Seulement / Diluer Avant
La Perfusion
Not for Direct Infusion /
Ne pa utilier pour perfuser directementHYPERTONIC / HYPERTONIQUE
CONTAINS NO MORE THAN 25 µg/l OF ALUMINUM /
NE CONTENT PAS PLUS DE 25 µg/l D’ALUMINIUMAPPROX mOsmol/L – 3530 APPROX pH 4.0
INTRAVENOUS FLUID AND NUTRIENT REPLENISHMENT /
RECHARGE LIQUIDIENNE ET NUTRIMENT PAR
INJECTION INTRAVEINEUSEPER 100 mL DEXTROSE HYDROUS USP – 70 g / WATER
FOR INJECTION USP qs
PAR 100 mL DEXTROSE HYDRATE USP – 70 g / EAU
POUR INJETION USP qsCOLOUR VARIATION FROM LIGHT YELLOW TO AMBER IS
NORMAL AND DOES NOT ALTER EFFICACY / IL EST NOR-
MAL QUE LA COULEUR VARIE D’UN JAUNE PALE A UN
JAUNE AMBRE ET CELA N’AFFECTE PAS L’EFFICACITEAFFIX ACCOMPANYING LABEL FOR DATE AND TIME OF
ENTRY / APPOSER UNE ETIQUETTE ET INSCRIRE LA
DATE ET L’HEURE DU PRELEVEMENT INITIAL / DISCARD
UNUSED CONTENTS WITHIN 4 HOURS OF INITIAL ENTRY
/ JETER LE CONTENANT 4 HEURES APRES LE PREMIER
PRELEVEMENTCAUTIONS SQUEEZE AND INSPECT BAG / SEE DIREC-
TIONS FOR USE / STORE AT 15°C TO 25°C
ATTENTIONS PRESSER ET INSPECTER LE SAC / VOIR
MODE D’EMPLOI / GARDER ENTIRE 15°C ET 25°CNONPYROGENIC / STERILE / APYROGENE / STERILE
PRESCRIBING INFORMATION AVAILABLE ON REQUEST / INFORMA-
TION POSOLOGIQUE DISPONIBLE SUR DEMANDEVIAFLEXPVC CONTAINER/CONTENANT DE PVC
BAXTER AND VIAFLEX ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
BAXTER ET VIAFLEX SONT DE MARQUES DE COMMERCE DE BAXTER
INTERNATIONAL INCBaxter Logo
BAXTER CORPORATION
Mississauga ON L5N 0C270%
No Latex Label
88-70-20-4622100
1800
1500
1200
900
600
300
JB-02-97
4 – 3000 mL units
Store at 15°C to 25°C70% Dextrose Injection USP
Lot:WWWWWWWWW EXP: 2099 99
2D Barcode
(01)20809080000658Pharmacy Bulk
-
INGREDIENTS AND APPEARANCE
DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-9789 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 70 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9789-04 4 in 1 CARTON 10/18/2024 1 NDC:0338-9789-01 3000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/18/2024 Labeler - Baxter Healthcare Company (005083209) Establishment Name Address ID/FEI Business Operations Baxter Corporation 205087968 analysis(0338-9789) , label(0338-9789) , manufacture(0338-9789) , sterilize(0338-9789) , pack(0338-9789)