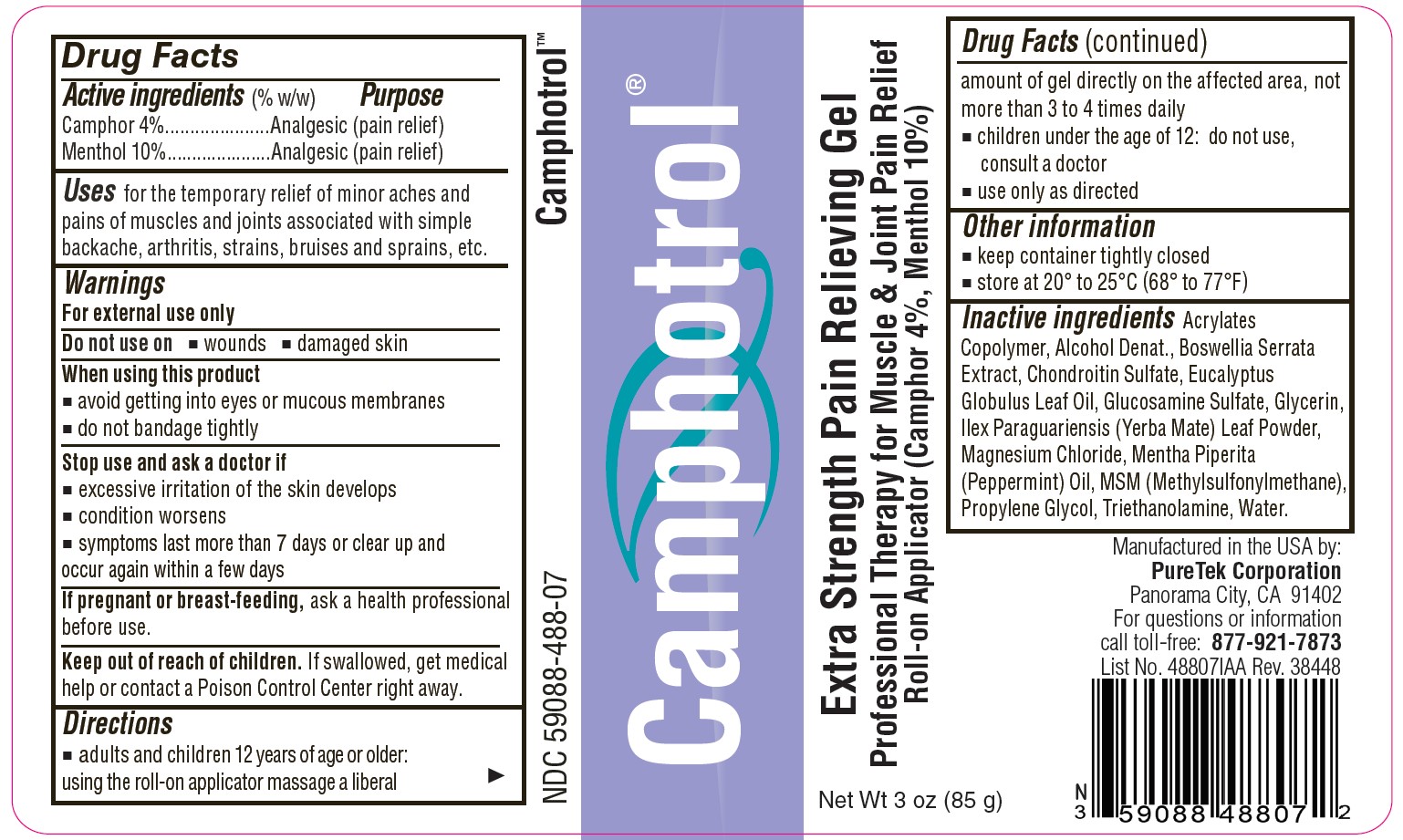
Label: CAMPHOTROL- camphor, menthol gel
- NDC Code(s): 59088-488-07
- Packager: PureTek Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (% by weight)
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Acrylates Copolymer, Alcohol Denat., Boswellia Serrata Extract, Chondroitin Sulfate, Eucalyptus Globulus Leaf Oil, Glucosamine Sulfate, Glycerin, Ilex Paraguariensis (Yerba Mate) Leaf Powder, Magnesium Chloride, Mentha Piperita (Peppermint) Oil, MSM (Methylsulfonylmethane), Propylene Glycol, Triethanolamine, Water.
- Camphotrol®
-
INGREDIENTS AND APPEARANCE
CAMPHOTROL
camphor, menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59088-488 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 4 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 g Inactive Ingredients Ingredient Name Strength METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (4500 MPA.S) (UNII: T967IEU43C) ALCOHOL (UNII: 3K9958V90M) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CHONDROITIN SULFATE (BOVINE) (UNII: 6IC1M3OG5Z) EUCALYPTUS OIL (UNII: 2R04ONI662) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) GLYCERIN (UNII: PDC6A3C0OX) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) PEPPERMINT OIL (UNII: AV092KU4JH) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-488-07 85 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 01/18/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/18/2022 Labeler - PureTek Corporation (785961046)