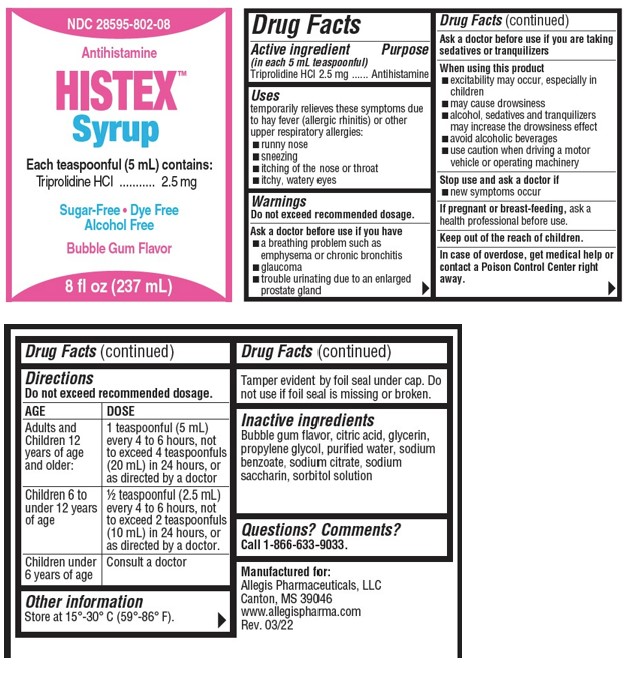
Label: HISTEX- triprolidine hydrochloride syrup
- NDC Code(s): 28595-802-08
- Packager: Allegis Pharmaceuticals, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each 5 mL teaspoonful)
- Purpose
- Uses
-
Warnings
Do not exceed recommended dosage.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor before use if you are taking sedatives or tranquilizers
-
Directions
Do not exceed recommended dosage.
AGE DOSE Adults and Childen 12 years of age and older: 1 teaspoonful (5 mL) every 4 to 6 hours, not to exceed 4 teaspoonfuls (20 mL) in 24 hours, or as directed by a doctor Childen 6 to under 12 years of age ½ teaspoonful (2.5 mL) every 4 to 6 hours, not to exceed 2 teaspoonfuls (10 mL) in 24 hours, or as directed by a doctor. Children under 6 years of age Consult a doctor - Other Information
- Inactive ingredients
- Questions? Comments?
- PRINCIPAL DISPLAY PANEL - 237 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
HISTEX
triprolidine hydrochloride syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:28595-802 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIPROLIDINE HYDROCHLORIDE (UNII: YAN7R5L890) (TRIPROLIDINE - UNII:2L8T9S52QM) TRIPROLIDINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:28595-802-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/04/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 03/04/2014 Labeler - Allegis Pharmaceuticals, LLC (792272861)