Label: COMFORT SHIELD BARRIER- dimethicone cloth
-
NDC Code(s):
53462-915-24,
53462-915-50,
53462-915-51,
53462-915-62, view more53462-915-80, 53462-915-81
- Packager: Sage Products LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
-
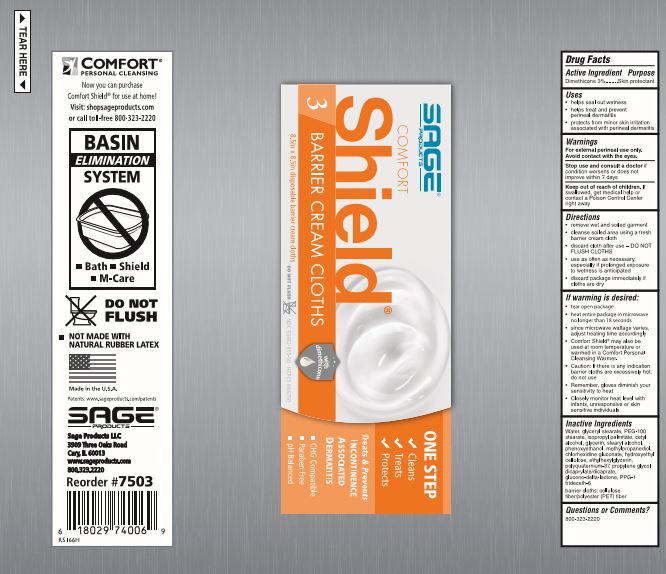
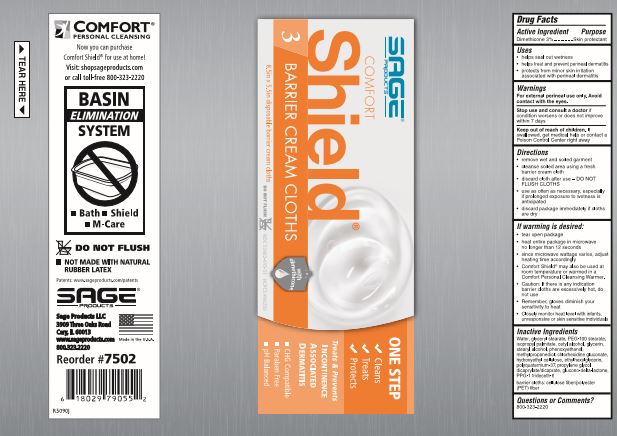
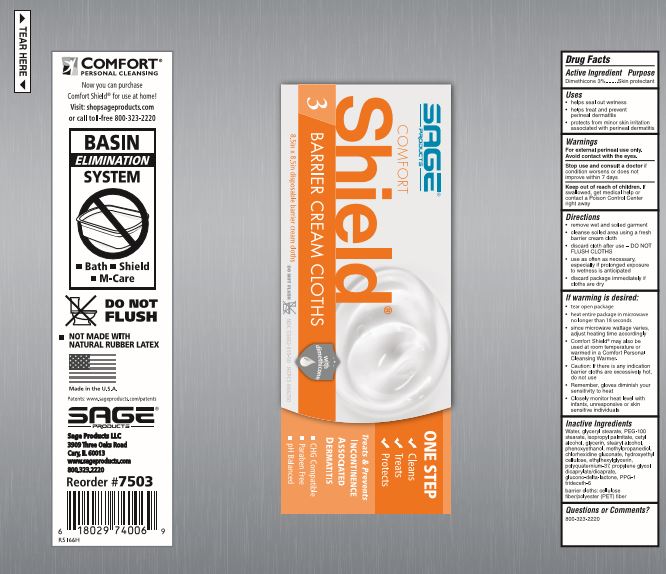
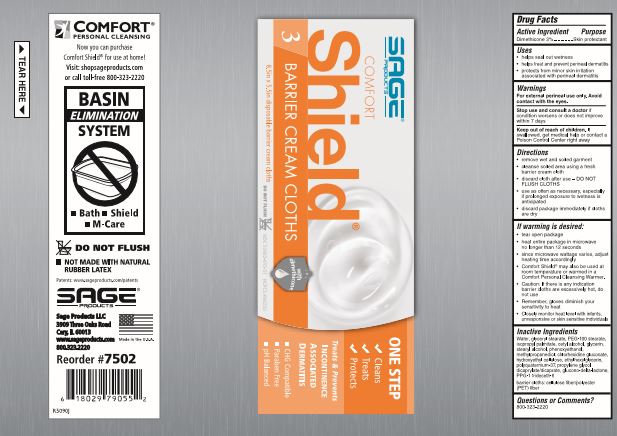
Directions
- remove wet and soiled garment
- cleanse soiled area using a fresh barrier cream cloth
- discard cloth after use - DO NOT FLUSH CLOTHS
- use as often as necessary, especially if prolonged exposure to wetness is anticipated
- for individual use only
- reseal label between uses
- discard package immediately if cloths are dry
If warming is desired:
- peel open label without completely removing it
- heat entire package in microwave no longer than [see package for time].
- since microwave wattage varies, adjust heating time accordingly
- Comfort Shield® may also be used at room temperature or warmed in a Comfort Personal Cleansing Warmer.
- Caution: If there is any indication barrier cloths are excessively hot, do not use
- Remember, gloves diminish your sensitivity to heat
- Closely monitor heat level with infants, unresponsive or skin sensitive individuals
-
Inactive Ingredients
Water, glyceryl stearate, PEG-100 stearate, isopropyl palmitate, cetyl alcohol, glycerin, stearyl alcohol, phenoxyethanol, methylpropanediol, chlorhexidine gluconate, hydroxyethyl cellulose, ethylhexylglycerin, polyquaternium-37, propylene glycol dicaprylate/dicaprate, glucono-delta-lactone, PPG-1 trideceth-6
- Questions or Comments?
- Comfort Shield Barrier
-
INGREDIENTS AND APPEARANCE
COMFORT SHIELD BARRIER
dimethicone clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53462-915 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) HYDROXYETHYL CELLULOSE (4000 MPA.S AT 1%) (UNII: ZYD53NBL45) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYQUATERNIUM-37 (25000 MPA.S) (UNII: 31L31U8285) PROPYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: O4446S9CRA) GLUCONOLACTONE (UNII: WQ29KQ9POT) PPG-1 TRIDECETH-6 (UNII: 1K7417JX6Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53462-915-50 3 in 1 PACKAGE 01/01/1990 1 26 g in 1 APPLICATOR; Type 0: Not a Combination Product 2 NDC:53462-915-51 3 in 1 PACKAGE 01/01/1990 2 19 g in 1 APPLICATOR; Type 0: Not a Combination Product 3 NDC:53462-915-62 24 in 1 PACKAGE 01/01/1990 09/21/2016 3 24 g in 1 APPLICATOR; Type 0: Not a Combination Product 4 NDC:53462-915-80 8 in 1 PACKAGE 01/01/1990 4 27 g in 1 APPLICATOR; Type 0: Not a Combination Product 5 NDC:53462-915-81 8 in 1 PACKAGE 01/01/1990 5 19 g in 1 APPLICATOR; Type 0: Not a Combination Product 6 NDC:53462-915-24 24 in 1 PACKAGE 12/11/2023 6 24 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 01/01/1990 Labeler - Sage Products LLC (054326178)