Label: NERD ACNE TREATMENT- sulfur lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 70425-101-30 - Packager: Panco Cosmetics Corp.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 14, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
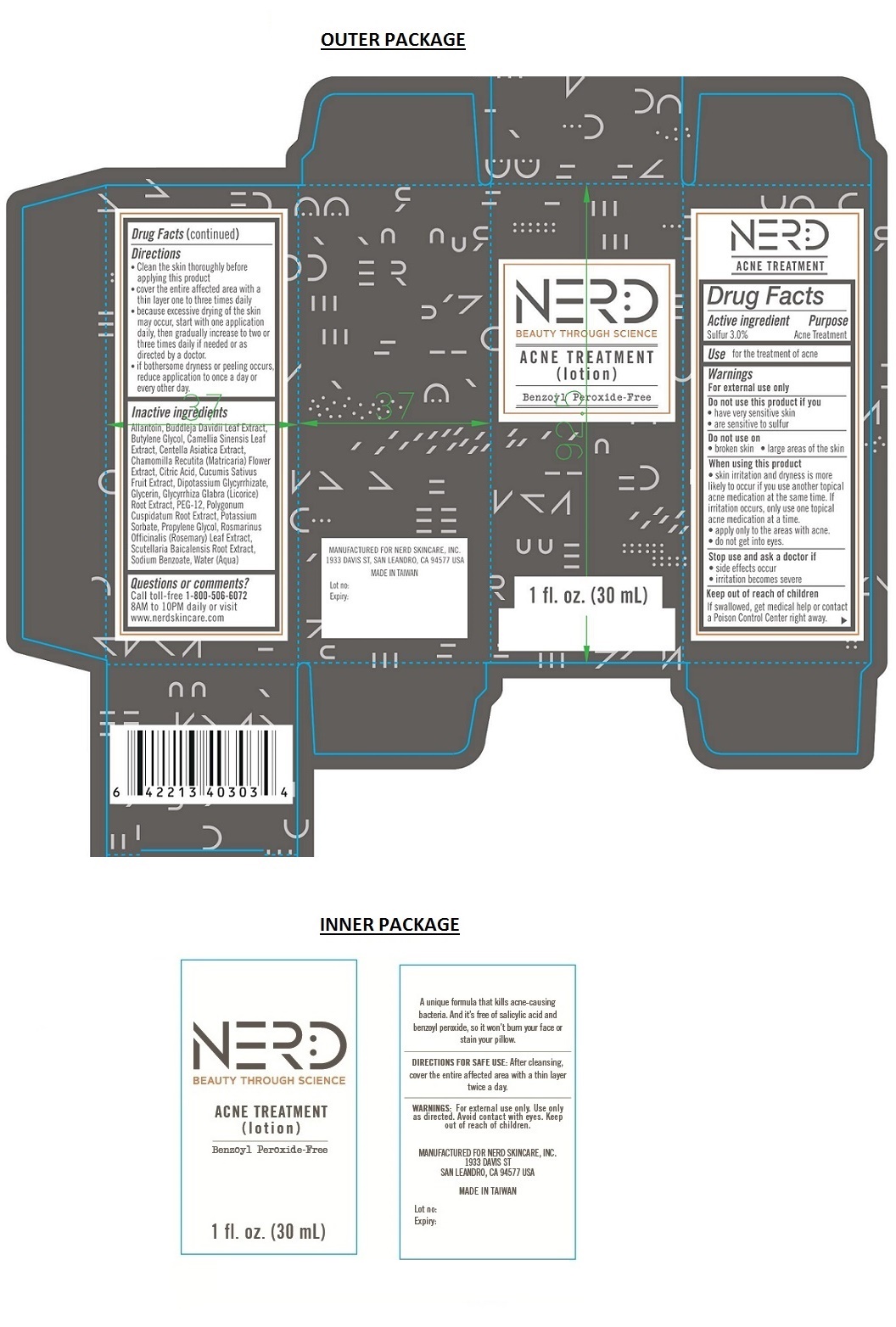
- Drug Facts
- Active ingredient
- Purpose
- Keep out of reach of children
- INDICATIONS & USAGE
-
Warnings
For external use only
Do not use this product if you
• have very sensitive skin
• are sensitive to sulfur
Do not use on
• broken skin • large areas of the skin
When using this product
• skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
• apply only to the areas with acne.
• do not get into eyes.
Stop use and ask a doctor if
• side effects occur
• irritation becomes severe
-
Directions
• Clean the skin thoroughly before applying this product
• cover the entire affected area with a thin layer one to three times daily
• because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
• if bothersome dryness or peeling occurs, reduce application to once a day or every other day. -
Inactive ingredients
Allantoin, Buddleja Davidii Leaf Extract, Butylene Glycol, Camellia Sinensis Leaf Extract, Centella Asiatica Extract, Chamomilla Recutita (Matricaria) Flower Extract, Citric Acid, Cucumis Sativus Fruit Extract, Dipotassium Glycyrrhizate, Glycerin, Glycyrrhiza Glabra (Licorice) Root Extract, PEG-12, Polygonum Cuspidatum Root Extract, Potassium Sorbate, Propylene Glycol, Rosmarinus Officinalis (Rosemary) Leaf Extract, Scutellaria Baicalensis Root Extract, Sodium Benzoate, Water (Aqua)
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
NERD ACNE TREATMENT
sulfur lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70425-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 0.9 g in 30 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) BUDDLEJA DAVIDII LEAF (UNII: X380815D32) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CENTELLA ASIATICA (UNII: 7M867G6T1U) CHAMOMILE (UNII: FGL3685T2X) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CUCUMBER (UNII: YY7C30VXJT) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) GLYCERIN (UNII: PDC6A3C0OX) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) POLYGONUM CUSPIDATUM ROOT (UNII: 7TRV45YZF7) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ROSEMARY (UNII: IJ67X351P9) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70425-101-30 1 in 1 BOX 03/01/2016 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 03/01/2016 Labeler - Panco Cosmetics Corp. (657836698) Establishment Name Address ID/FEI Business Operations Panco Cosmetics Corp. 657836698 manufacture(70425-101)