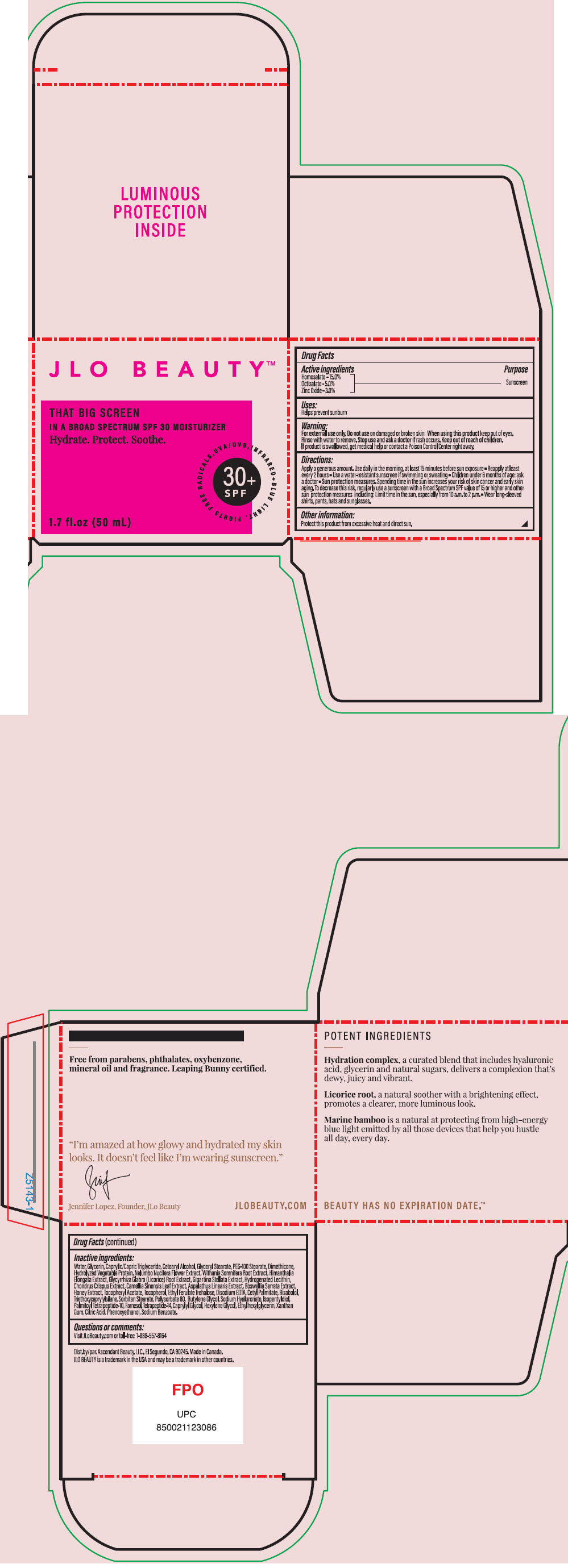
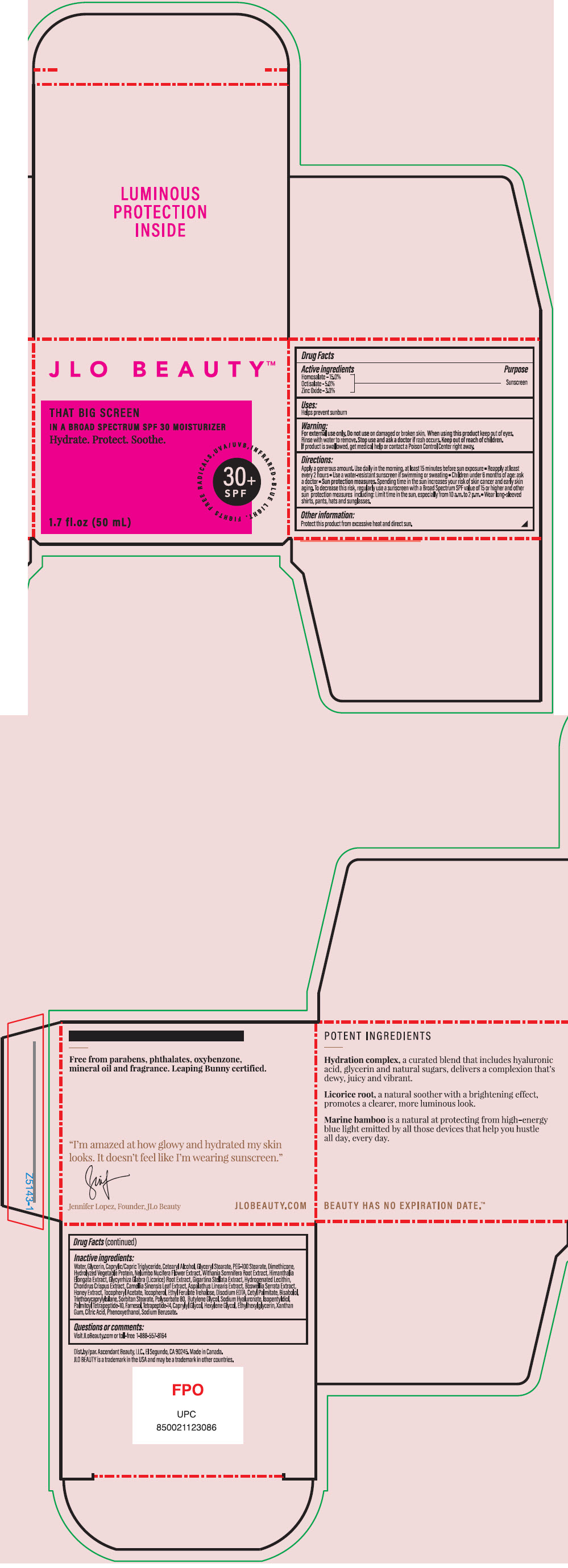
Label: JLO BEAUTY THAT BIG SCREEN BROAD SPECTRUM SPF 30 MOISTURIZER- homosalate, octisalate, and zinc oxide cream
- NDC Code(s): 79625-001-07, 79625-001-19
- Packager: Ascendant Beauty LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
Apply a generous amount. Use daily in the morning. At least 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimming or sweating
- Children under 6 months of age: ask a doctor
- Sun protection measures, Spending time in the sun increases your risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Limit in the sun, especially from 10 a.am. to 2 p.m.
- Wear long0sleeved shirts, pants, hats and sunglasses.
- Other information
- Active Ingredients
-
Inactive Ingredients
Water, Glycerin, Caprylic/Capric Triglyceride, Cetearyl Alcohol, Glyceryl Stearate, PEG-100 Stearate, Dimethicone, Hydrolyzed Vegetable Protein, Nelumbo Nucifera Flower Extract, Withania Somnifera Root Extract, Himanthalia Elongata Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Gigartina Stellata Extract, Hydrogenated Lecithin, Chondrus Crispus Extract, Camellia Sinensis Leaf Extract, Aspalathus Linearis Extract, Boswellia Serrata Extract, Honey Extract, Tocopheryl Acetate, Tocopherol, Ethyl Ferulate Trehalose, Disodium EDTA, Cetyl Palmitate, Bisabolol, Triethoxycaprylylsilane, Sorbitan Stearate, Polysorbate 80, Butylene Glycol, Sodium Hyaluronate, Isopentyldiol, Palmitoyl Tetrapeptide-10, Farnesol, Tetrapeptide-14, Caprylyl Glycol, Hexylene Glycol, Ethylhexylglycerin, Xanthan Gum, Citric Acid, Phenoxyethanol, Sodium Benzoate.
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 mL Jar Box
-
INGREDIENTS AND APPEARANCE
JLO BEAUTY THAT BIG SCREEN BROAD SPECTRUM SPF 30 MOISTURIZER
homosalate, octisalate, and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79625-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 150 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 Stearate (UNII: YD01N1999R) Dimethicone (UNII: 92RU3N3Y1O) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) WITHANIA SOMNIFERA ROOT (UNII: V038D626IF) HIMANTHALIA ELONGATA (UNII: 21RND18XRR) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) MASTOCARPUS STELLATUS (UNII: 6T087FC66H) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) GREEN TEA LEAF (UNII: W2ZU1RY8B0) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) HONEY (UNII: Y9H1V576FH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Tocopherol (UNII: R0ZB2556P8) ETHYL FERULATE (UNII: 5B8915UELW) TREHALOSE (UNII: B8WCK70T7I) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Cetyl Palmitate (UNII: 5ZA2S6B08X) LEVOMENOL (UNII: 24WE03BX2T) Triethoxycaprylylsilane (UNII: LDC331P08E) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) Polysorbate 80 (UNII: 6OZP39ZG8H) Butylene Glycol (UNII: 3XUS85K0RA) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Isopentyldiol (UNII: 19NOL5474Q) Palmitoyl Tetrapeptide-10 (UNII: 6Q6H1PKC05) Farnesol (UNII: EB41QIU6JL) Caprylyl Glycol (UNII: 00YIU5438U) Hexylene Glycol (UNII: KEH0A3F75J) Ethylhexylglycerin (UNII: 147D247K3P) Xanthan Gum (UNII: TTV12P4NEE) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Phenoxyethanol (UNII: HIE492ZZ3T) Sodium Benzoate (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79625-001-07 15 mL in 1 JAR; Type 0: Not a Combination Product 04/01/2021 2 NDC:79625-001-19 1 in 1 BOX 04/01/2021 2 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 04/01/2021 Labeler - Ascendant Beauty LLC (117586353)