Label: NIFEDIPINE tablet, extended release
- NDC Code(s): 72162-1887-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 68682-105
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - For Oral Use
-
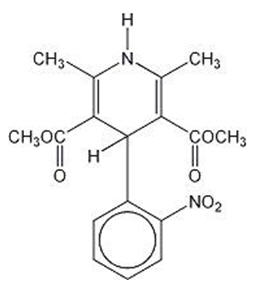
DESCRIPTIONNifedipine extended-release tablets are an extended-release tablet dosage form of the calcium channel blocker nifedipine. Nifedipine is 3,5-pyridinedicarboxylic acid ...
-
CLINICAL PHARMACOLOGYNifedipine is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist) which inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac ...
-
INDICATIONS AND USAGENifedipine extended-release tablets are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
-
CONTRAINDICATIONSConcomitant administration with strong P450 inducers, such as rifampin, are contraindicated since the efficacy of nifedipine tablets could be significantly reduced. (See PRECAUTIONS: Drug ...
-
WARNINGSExcessive Hypotension - Although in most patients the hypotensive effect of nifedipine is modest and well tolerated, occasional patients have had excessive and poorly tolerated hypotension. These ...
-
PRECAUTIONSGeneral - Hypotension - Because nifedipine decreases peripheral vascular resistance, careful monitoring of blood pressure during the initial administration and titration of nifedipine ...
-
ADVERSE REACTIONSThe incidence of adverse events during treatment with nifedipine extended-release tablets in doses up to 90 mg daily were derived from multi-center placebo-controlled clinical trials in 370 ...
-
OVERDOSAGEExperience with nifedipine overdosage is limited. Symptoms associated with severe nifedipine overdosage include loss of consciousness, drop in blood pressure, heart rhythm disturbances, metabolic ...
-
DOSAGE AND ADMINISTRATIONDosage should be adjusted according to each patient’s needs. It is recommended that nifedipine extended-release tablet be administered orally once daily on an empty stomach. Nifedipine ...
-
HOW SUPPLIEDNifedipine Extended-Release Tablets, USP are supplied as 30 mg mustard yellow, unscored, round, film-coated tablets, engraved with "B" on one side and "30" on the other side. NDC 72162-1887-2 ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Oceanside Pharmaceuticals, a division of - Bausch Health US, LLC - Bridgewater, NJ 08807 USA - Manufactured by: Bausch Health Companies Inc. Steinbach, MB R5G 1Z7, Canada - © 2019 ...
-
PRINCIPAL DISPLAY PANELNIFEdipine Extended-Release 30 mg Tablets
-
INGREDIENTS AND APPEARANCEProduct Information