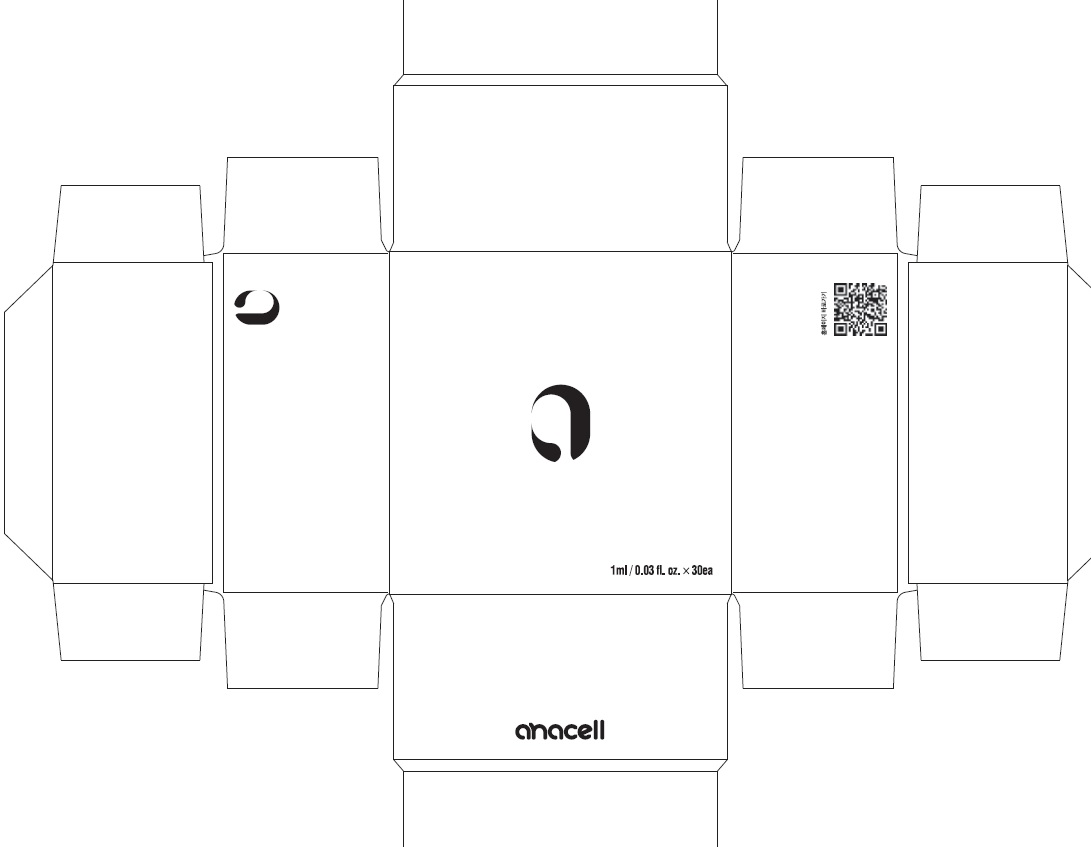
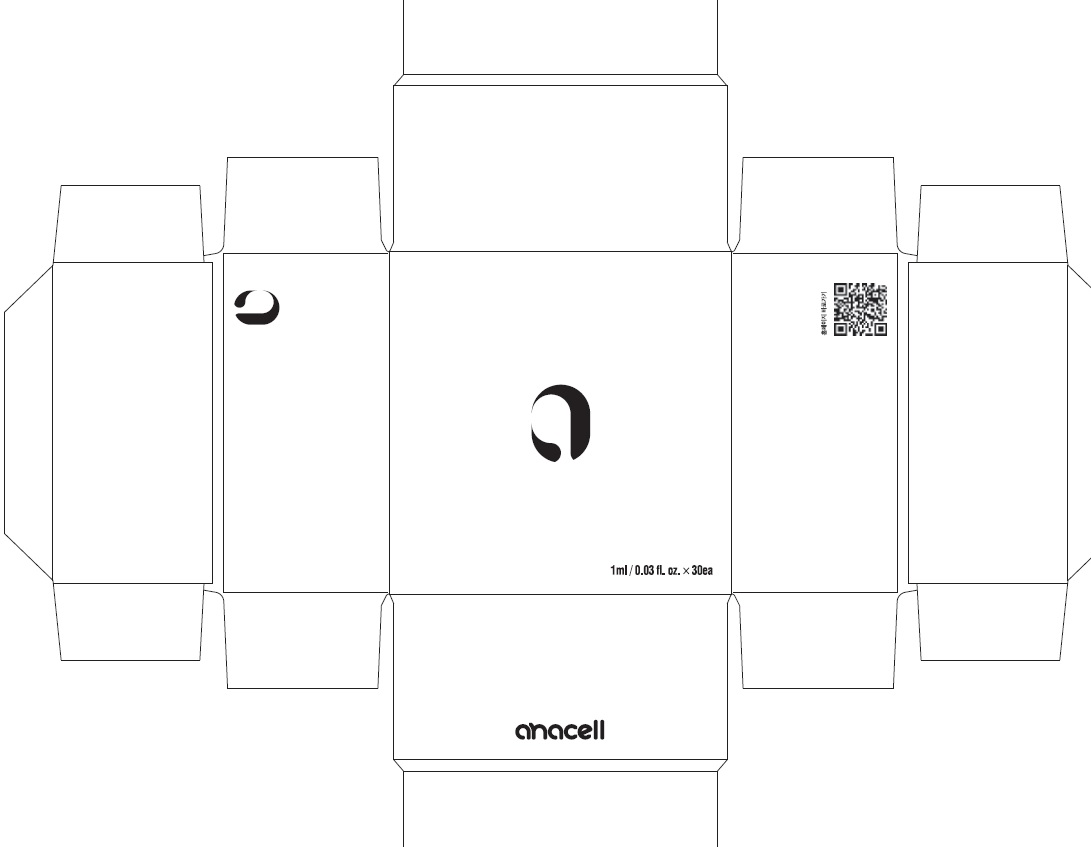
Label: ANACELL- centipeda minima whole liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 81823-010-01, 81823-010-02 - Packager: D-Nature Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 6, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENTS
- PURPOSE
-
WARNINGS
For external use only.
In case of contact with eyes, wash immediately.
In case of using cosmetics or after use, please consult with a specialist if there is any abnormal symptom or side effect such as red spot, swelling, or itching by direct sunlight.
Please do not use the wound area.
Store at room temperature.
Keep out of direct sunlight.
Keep out of reach of children. - KEEP OUT OF REACH OF CHILDREN
- Uses
-
Directions
After shampooing hair, dry it well and shaking the product sufficiently, apply an appropriate amount evenly to the scalp and rub it to absorb enough.
The best usage time is 3 to 8 hours.
Do not use for more than 8 hours.
Wash off with shampoo after use.
**Use the electric vibration hair massage device to maximize results. (5 to 10 minutes)** - QUESTIONS
- PACKAGE LABEL (Front)
- PACKAGE LABEL (Back, Drug Facts)
-
INGREDIENTS AND APPEARANCE
ANACELL
centipeda minima whole liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81823-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CENTIPEDA MINIMA WHOLE (UNII: 7F196Q97IQ) (CENTIPEDA MINIMA WHOLE - UNII:7F196Q97IQ) CENTIPEDA MINIMA WHOLE 47.5 g in 100 mL Inactive Ingredients Ingredient Name Strength CAPRYLOCAPROYL POLYOXYLGLYCERIDES 8 (UNII: 00BT03FSO2) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) Panthenol (UNII: WV9CM0O67Z) LAVENDER OIL (UNII: ZBP1YXW0H8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81823-010-02 30 in 1 CARTON 06/01/2021 1 NDC:81823-010-01 1 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/01/2021 Labeler - D-Nature Co., Ltd. (694822253) Registrant - D-Nature Co., Ltd. (694822253) Establishment Name Address ID/FEI Business Operations D-Nature Co., Ltd. 694822253 manufacture(81823-010)