Label: CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE tablet
-
NDC Code(s):
70771-1325-0,
70771-1325-1,
70771-1325-2,
70771-1325-3, view more70771-1325-4, 70771-1325-9, 70771-1326-0, 70771-1326-1, 70771-1326-2, 70771-1326-3, 70771-1326-4, 70771-1326-9, 70771-1327-0, 70771-1327-1, 70771-1327-2, 70771-1327-3, 70771-1327-4, 70771-1327-9
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 13, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
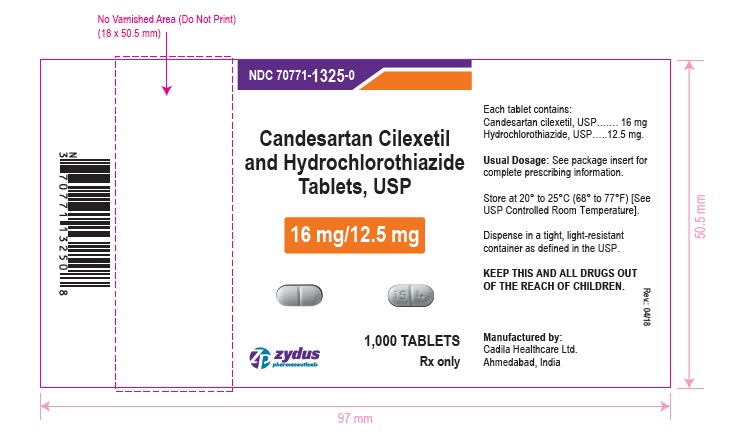
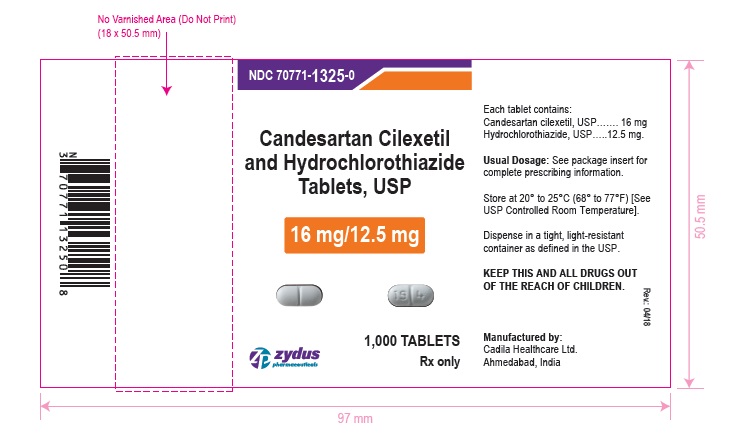
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1325-0 in bottle of 1000 tablets
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP, 16 mg/12.5 mg
Rx only
1000 tablets
NDC 70771-1326-0 in bottle of 1000 tablets
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP, 32 mg/12.5 mg
Rx only
1000 tablets

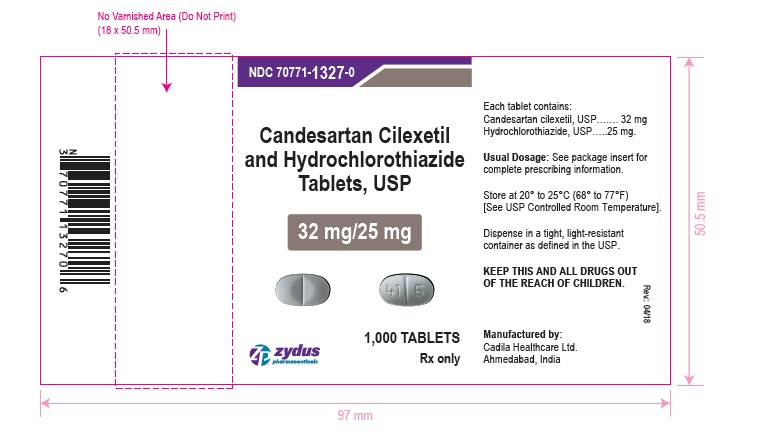
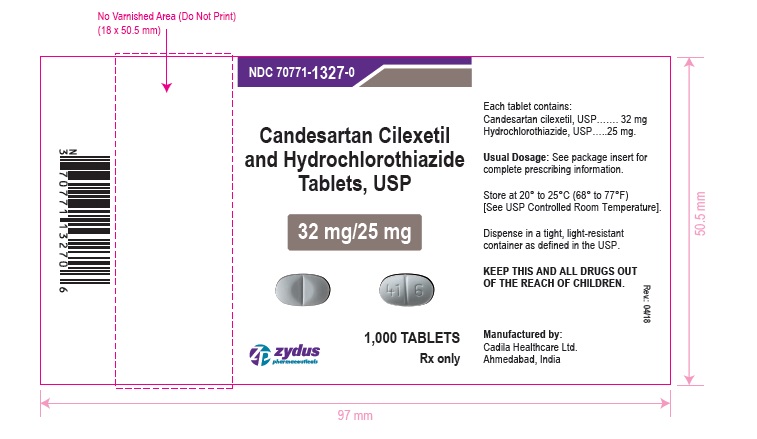
NDC 70771-1327-0 in bottle of 1000 tablets
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP, 32 mg/25 mg
Rx only
1000 tablets
-
INGREDIENTS AND APPEARANCE
CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
candesartan cilexetil and hydrochlorothiazide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1325 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 16 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) STARCH, CORN (UNII: O8232NY3SJ) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score 2 pieces Shape OVAL (OVAL) Size 10mm Flavor Imprint Code 19;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1325-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 2 NDC:70771-1325-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 3 NDC:70771-1325-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 4 NDC:70771-1325-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 5 NDC:70771-1325-4 10 in 1 CARTON 04/14/2018 5 NDC:70771-1325-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203466 04/14/2018 CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
candesartan cilexetil and hydrochlorothiazide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1326 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 32 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) STARCH, CORN (UNII: O8232NY3SJ) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score 2 pieces Shape OVAL (OVAL) Size 11mm Flavor Imprint Code 19;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1326-4 10 in 1 CARTON 04/14/2018 1 NDC:70771-1326-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:70771-1326-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 3 NDC:70771-1326-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 4 NDC:70771-1326-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 5 NDC:70771-1326-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203466 04/14/2018 CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
candesartan cilexetil and hydrochlorothiazide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1327 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 32 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 25 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) STARCH, CORN (UNII: O8232NY3SJ) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score 2 pieces Shape OVAL (OVAL) Size 11mm Flavor Imprint Code 41;6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1327-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 2 NDC:70771-1327-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 3 NDC:70771-1327-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 4 NDC:70771-1327-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2018 5 NDC:70771-1327-4 10 in 1 CARTON 04/14/2018 5 NDC:70771-1327-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203466 04/14/2018 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(70771-1325, 70771-1326, 70771-1327) , MANUFACTURE(70771-1325, 70771-1326, 70771-1327)