Label: FRESH CLEAR- phenylephrine hydrochloride solution/ drops
- NDC Code(s): 0023-0000-01, 0023-0000-02
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated February 1, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
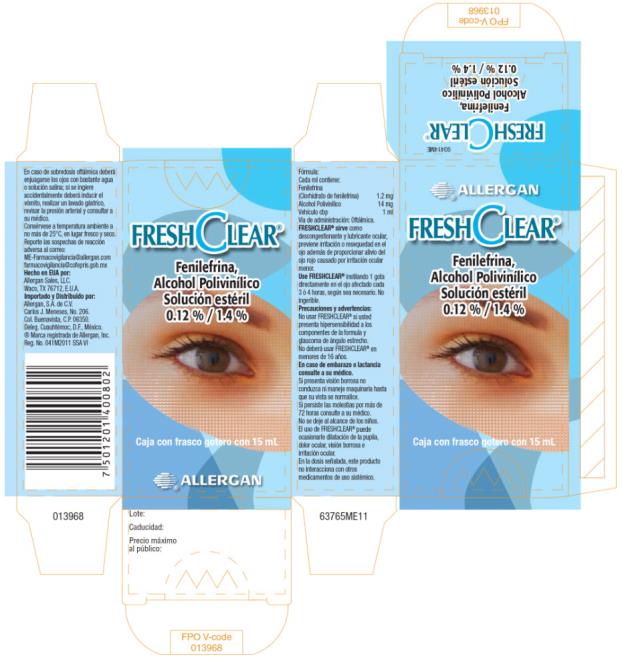
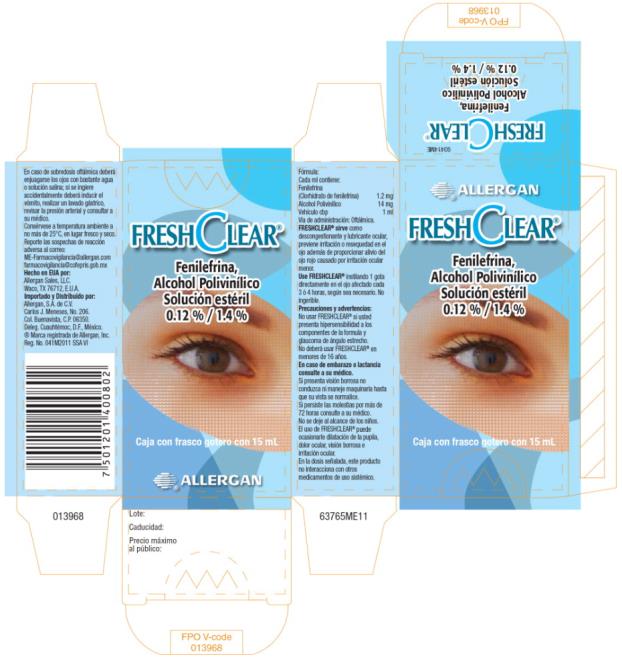
- Principal Display Panel- 1.2% / 1.4% 15 mL Carton Label
- Principal Display Panel - 0.12% 15 mL Carton Label
-
INGREDIENTS AND APPEARANCE
FRESH CLEAR
phenylephrine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0023-0000 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength phenylephrine hydrochloride (UNII: 04JA59TNSJ) (phenylephrine - UNII:1WS297W6MV) phenylephrine hydrochloride 1.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength edetate disodium (UNII: 7FLD91C86K) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) sodium phosphate, dibasic, heptahydrate (UNII: 70WT22SF4B) sodium phosphate, monobasic, monohydrate (UNII: 593YOG76RN) sodium acetate (UNII: 4550K0SC9B) sodium thiosulfate (UNII: HX1032V43M) water (UNII: 059QF0KO0R) benzalkonium chloride (UNII: F5UM2KM3W7) hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0023-0000-01 1 in 1 CARTON 11/01/2011 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:0023-0000-02 1 in 1 CARTON 11/01/2011 2 3 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 11/01/2011 Labeler - Allergan, Inc. (144796497)